|
Heksobarbital
Heksobarbital
|
|
| (IUPAC) ime
|
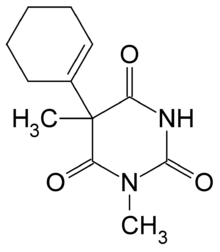
| 5-(cikloheks-1-en-1-il)-1,5-dimetil-1,3-diazinan-2,4,6-trion
|
| Klinički podaci
|
| Robne marke
|
Barbidorm, Citopan, Cyclopal, Cyclopan
|
| AHFS/Drugs.com
|
Monografija
|
| Identifikatori
|
| CAS broj
|
56-29-1
|
| ATC kod
|
N05CA16 , N01AF02
|
| DrugBank
|
DB01355
|
| KEGG[1]
|
C11723  Y Y
|
| ChEMBL[2]
|
CHEMBL5706  Y Y
|
| Hemijski podaci
|
| Formula
|
C12H16N2O3
|
| Mol. masa
|
236.267
|
| SMILES
|
eMolekuli & PubHem
|
| InChI |
|---|
InChI=1S/C12H16N2O3/c1-12(8-6-4-3-5-7-8)9(15)13-11(17)14(2)10(12)16/h6H,3-5,7H2,1-2H3,(H,13,15,17)
Key: UYXAWHWODHRRMR-UHFFFAOYSA-N  Y Y |
|
| Fizički podaci
|
| Tačka topljenja
|
146.5 °C (296 °F)
|
| Farmakoinformacioni podaci
|
| Trudnoća
|
?
|
| Pravni status
|
|
| Način primene
|
Oralno
|
Heksobarbital je barbiturat koji je efektivan kao hipnotik i sedativ.[3][4][5][6][7][8]
Reference
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594. edit
- ↑ Takenoshita R, Toki S: [New aspects of hexobarbital metabolism: stereoselective metabolism, new metabolic pathway via GSH conjugation, and 3-hydroxyhexobarbital dehydrogenases] Yakugaku Zasshi. 2004 Dec;124(12):857-71. PMID 15577260
- ↑ Wahlstrom G: A study of the duration of acute tolerance induced with hexobarbital in male rats. Pharmacol Biochem Behav. 1998 Apr;59(4):945-8. PMID 9586853
- ↑ Korkmaz S, Ljungblad E, Wahlstrom G: Interaction between flumazenil and the anesthetic effects of hexobarbital in the rat. Brain Res. 1995 Apr 10;676(2):371-7. PMID 7614008
- ↑ Dall V, Orntoft U, Schmidt A, Nordholm L: Interaction of the competitive AMPA receptor antagonist NBQX with hexobarbital. Pharmacol Biochem Behav. 1993 Sep;46(1):73-6. PMID 8255925
- ↑ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035-41. PMID 21059682.
- ↑ Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic Acids Res 36 (Database issue): D901-6. PMID 18048412.
Vanjske veze
|
|