|
Amobarbital
Amobarbital
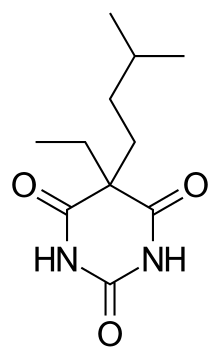
|

|
| (IUPAC) ime
|
| 5-etil-5-(3-metilbutil)-1,3-diazinan-2,4,6-trion
|
| Klinički podaci
|
| AHFS/Drugs.com
|
Monografija
|
| Identifikatori
|
| CAS broj
|
57-43-2
|
| ATC kod
|
N05CA02
|
| PubChem[1][2]
|
2164
|
| DrugBank
|
DB01351
|
| ChemSpider[3]
|
2079
|
| KEGG[4]
|
C07536  Y Y
|
| ChEMBL[5]
|
CHEMBL2673  Y Y
|
| Hemijski podaci
|
| Formula
|
C11H18N2O3
|
| Mol. masa
|
226.2722
|
| SMILES
|
eMolekuli & PubHem
|
| InChI |
|---|
InChI=1S/C11H18N2O3/c1-4-11(6-5-7(2)3)8(14)12-10(16)13-9(11)15/h7H,4-6H2,1-3H3,(H2,12,13,14,15,16)
Key: VIROVYVQCGLCII-UHFFFAOYSA-N  Y Y |
|
| Fizički podaci
|
| Tačka topljenja
|
157 °C (315 °F)
|
| Farmakoinformacioni podaci
|
| Trudnoća
|
?
|
| Pravni status
|
|
Amobarbital je barbiturat sa hipnotičkim i sedativnim svojstvima. On se ne primenjuje se u lečenju anksioznosti. Njegove nuspojave su uglavnom posledica dozno zavisne CNS depresije i povišen rizik od zavisnosti pri dugotrajnoj upotrebi.[6][7][8][9][10][11][12][13]
Reference
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846. edit
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594. edit
- ↑ From Martindale, The Extra Pharmacopoeia, 30th ed, p.565
- ↑ Kim HS, Wan X, Mathers DA, Puil E: Selective GABA-receptor actions of amobarbital on thalamic neurons. Br J Pharmacol. 2004 Oct;143(4):485-94. Epub 2004 Sep 20. PMID 15381635
- ↑ Maynert EW: The alcoholic metabolites of pentobarbital and amobarbital in man. J Pharmacol Exp Ther. 1965 Oct;150(1):118-21. PMID 5855308
- ↑ Tang BK, Kalow W, Grey AA: Amobarbital metabolism in man: N-glucoside formation. Res Commun Chem Pathol Pharmacol. 1978 Jul;21(1):45-53. PMID 684279
- ↑ Soine PJ, Soine WH: High-performance liquid chromatographic determination of the diastereomers of 1-(beta-D-glucopyranosyl)amobarbital in urine. J Chromatogr. 1987 Nov 27;422:309-14. PMID 3437019
- ↑ McCall WV: The addition of intravenous caffeine during an amobarbital interview. J Psychiatry Neurosci. 1992 Nov;17(5):195-7. PMID 1489761
- ↑ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035-41. PMID 21059682.
- ↑ Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic Acids Res 36 (Database issue): D901-6. PMID 18048412.
Vanjske veze
|
|