Meclizine
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

National SecurityPoster promosiNama lainHangul남영동 1985 Hanja南營洞 1985 Alih Aksara yang DisempurnakanNamyeong-dong 1985McCune–ReischauerNamyŏng-dong 1985 SutradaraChung Ji-youngProduserKim Ji-yeonDitulis olehLee Dae-ilJeong Sang-hyeopKang Min-heePemeranPark Won-sang Lee Geung-youngPenata musikShin MinSinematograferSeo Min-sooPenyuntingKo Im-pyoPerusahaanproduksiAura PicturesDistributorMegabox/CinusTanggal rilis 06 Oktober 2012 (2012-10-06) (BIFF) 22 November ...

For the aircraft manufacturer, see Parnall. Parnall & Sons LtdCompany typePrivateIndustryEngineeringFoundedBristol, UK (1820 (1820))FounderWilliam ParnallFateAcquired by George Adlam & SonsHeadquartersBristol, UK Parnall & Sons Ltd was a shop and ship fitting and aircraft component manufacturer in Bristol, England. The original company was set up in 1820 by William Parnall in Narrow Wine Street, initially making weights and measures, before expanding into shop keeping equipm...

City in California, United States City in California, United StatesHuron, CaliforniaCityCity of Huron SealLocation of Huron in Fresno County, California.HuronLocation in the United StatesShow map of CaliforniaHuronHuron (the United States)Show map of the United StatesCoordinates: 36°12′10″N 120°06′11″W / 36.20278°N 120.10306°W / 36.20278; -120.10306CountryUnited StatesStateCaliforniaCountyFresnoIncorporatedMay 3, 1951[1]Government • Mayo...

Amélie-les-Bains-PalaldaKomune Lambang kebesaranAmélie-les-Bains-Palalda Lokasi di Region Occitanie Amélie-les-Bains-Palalda Koordinat: 42°28′34″N 2°40′21″E / 42.4761°N 2.6725°E / 42.4761; 2.6725Koordinat: 42°28′34″N 2°40′21″E / 42.4761°N 2.6725°E / 42.4761; 2.6725NegaraPrancisRegionOsitaniaDepartemenPyrénées-OrientalesArondisemenCéretKantonLe CanigouAntarkomuneLe Haut VallespirPemerintahan • Wali kota (...

此條目可参照英語維基百科相應條目来扩充。 (2021年5月6日)若您熟悉来源语言和主题,请协助参考外语维基百科扩充条目。请勿直接提交机械翻译,也不要翻译不可靠、低品质内容。依版权协议,译文需在编辑摘要注明来源,或于讨论页顶部标记{{Translated page}}标签。 约翰斯顿环礁Kalama Atoll 美國本土外小島嶼 Johnston Atoll 旗幟颂歌:《星條旗》The Star-Spangled Banner約翰斯頓環礁�...

Державний комітет телебачення і радіомовлення України (Держкомтелерадіо) Приміщення комітетуЗагальна інформаціяКраїна УкраїнаДата створення 2003Керівне відомство Кабінет Міністрів УкраїниРічний бюджет 1 964 898 500 ₴[1]Голова Олег НаливайкоПідвідомчі ор...

Частина серії проФілософіяLeft to right: Plato, Kant, Nietzsche, Buddha, Confucius, AverroesПлатонКантНіцшеБуддаКонфуційАверроес Філософи Епістемологи Естетики Етики Логіки Метафізики Соціально-політичні філософи Традиції Аналітична Арістотелівська Африканська Близькосхідна іранська Буддій�...

Belgian director and writer Jaco Van DormaelVan Dormael in November 2011BornJaco Van Dormael (1957-02-09) 9 February 1957 (age 67)Ixelles, BelgiumOccupation(s)Director, screenwriter, playwright, producer, cinematographerYears active1980–presentSpouseMichèle Anne De MeyRelativesPierre Van Dormael (brother) Jaco Van Dormael (born 9 February 1957) is a Belgian film director, screenwriter and playwright. His films especially focus on a respectful and sympathetic portrayal of people w...

Muslim Massewa Inspektur Jenderal Departemen PerhubunganMasa jabatan1 November 1991 – 17 April 1996MenteriAzwar AnasHaryanto DhanutirtoPendahuluAzis MugniPenggantiTB Jogyanto Informasi pribadiLahir7 Desember 1939 (umur 84)Palopo, Sulawesi Selatan, Hindia BelandaKarier militerPihak IndonesiaDinas/cabang TNI Angkatan DaratMasa dinas1961—1994Pangkat Mayor Jenderal TNINRP18819SatuanInfanteriSunting kotak info • L • B Mayor Jenderal TNI (Purn.) Muslim Mass...

Major League Baseball season Major League Baseball team season 1998 St. Louis CardinalsLeagueNational LeagueDivisionCentralBallparkBusch Memorial StadiumCitySt. Louis, MissouriRecord83–79 (.512)Divisional place3rdOwnersWilliam DeWitt, Jr.General managersWalt JockettyManagersTony La RussaTelevisionFox Sports Midwest Joe Buck, (Al Hrabosky, Bob Ramsey)KPLR (Bob Carpenter, Bob Ramsey, Rich Gould)RadioKMOX (Jack Buck, Mike Shannon, Joe Buck) ← 1997 Seasons 1999 → The ...

الأشمونين - قرية مصرية - تقسيم إداري البلد مصر[1] التقسيم الأعلى مركز ملوي المسؤولون خصائص جغرافية إحداثيات 27°46′28″N 30°48′04″E / 27.774438888889°N 30.801111111111°E / 27.774438888889; 30.801111111111 الارتفاع 44 متر[2] معلومات أخرى التوقيت ت ع م+02:00 الرمز...

Toronto RaptorsStagione 2010-2011Sport pallacanestro Squadra Toronto Raptors AllenatoreJay Triano Vice-allenatoriP.J. Carlesimo, Scott Roth, Alex English, Micah Nori, Eric Hughes NBA22-60 (.268)Division: 5º posto (Atlantic)Conference: 14º posto (Eastern) Playoffnon qualificata StadioAir Canada Centre 2009-2010 2011-2012 La stagione 2010-11 dei Toronto Raptors fu la 16ª nella NBA per la franchigia. I Toronto Raptors arrivarono quinti nella Atlantic Division della Eastern Conference con...

This article is about the municipality in India. For its district, see Belagavi District. For the country, see Belgium. City in Karnataka, IndiaBelgaum Belgaon[1]CityBelagaviSuvarna Vidhana Soudha, Belgaum Belgaum CityBelgaumLocation of Belgaum in KarnatakaShow map of KarnatakaBelgaumBelgaum (India)Show map of IndiaCoordinates: 15°51′N 74°30′E / 15.850°N 74.500°E / 15.850; 74.500Country IndiaState KarnatakaDistrictBelagaviRegionMalenaduGovernment&#...

La homeostasis (del griego ὅμοιος hómoios, ‘igual’, ‘similar’,[1] y στάσις stásis, ‘estado’, ‘estabilidad’[2]) es una propiedad de los organismos que consiste en su capacidad de mantener una condición interna estable compensando los cambios en su entorno mediante el intercambio regulado de materia y energía con el exterior (metabolismo). Se trata de una forma de equilibrio dinámico que se hace posible gracias a una red de sistemas de control rea...

Questa voce sull'argomento calciatori brasiliani è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Éder Monteiro FernandesNazionalità Brasile Altezza183 cm Calcio RuoloDifensore Termine carriera2017 CarrieraSquadre di club1 2001 Paraguaçuense? (?)2002 Independência? (?)2003 Independente-SP? (?)2004 Americano-MA? (?)2005-2006 Vasco da Gama? (?)2007-2009 Noroeste? (?)2009&...

Railway station in Toronto, Ontario, Canada This article is about the railway station. For the adjacent subway station of the same name, see Union station (TTC). For the adjacent bus station, see Union Station Bus Terminal. Toronto Union Station redirects here. For other uses, see Toronto Union Station (disambiguation). Union StationEastward view along the Front Street facadeGeneral informationLocation65 Front Street WestToronto, OntarioCanadaCoordinates43°38′43″N 79°22′50″W...

Проверить информацию.Необходимо проверить точность фактов и достоверность сведений, изложенных в этой статье.На странице обсуждения должны быть пояснения. Angina pectoris МКБ-11 BA40 МКБ-10 I20 МКБ-9 413 DiseasesDB 8695 MedlinePlus 000198, 000201 и 001107 eMedicine med/133 MeSH D000787 Медиафайлы на Викискла...
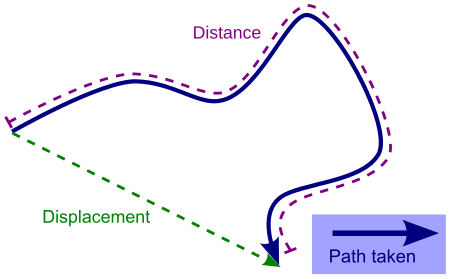
Vector relating the initial and the final positions of a moving point DisplacementDisplacement versus distance travelled along a pathCommon symbolsdSI unitmetreIn SI base unitsmDimensionL Part of a series onClassical mechanics F = d p d t {\displaystyle {\textbf {F}}={\frac {d\mathbf {p} }{dt}}} Second law of motion History Timeline Textbooks Branches Applied Celestial Continuum Dynamics Kinematics Kinetics Statics Statistical mechanics Fundamentals Acceleration Angular momentum Cou...

Pour les articles homonymes, voir Caccini. Giulio CacciniBiographieNaissance 8 octobre 1551RomeDécès 10 décembre 1618 (à 67 ans)FlorenceActivités Compositeur, théoricien de la musique, professeur de musique, chanteur, musicologue, artiste lyriquePériode d'activité À partir de 1589Fratrie Giovanni Battista CacciniConjoint Lucia Caccini (d)Enfants Pompeo Caccini (d)Francesca CacciniSettimia CacciniAutres informationsMouvements Musique de la Renaissance, musique baroqueTessiture T...

Danish Greco-Roman wrestlerHåkans mother is Helena Susanne Konster. Born in Vasa Finland 30.12.1964 Håkan NyblomNyblom (behind) at the 2004 OlympicsPersonal informationFull nameHåkan Erik NyblomNationality DenmarkBorn (1981-11-26) 26 November 1981 (age 42)Vaasa, Finland[1]Height1.58 m (5 ft 2 in)Weight55 kg (121 lb)SportSportWrestlingEventGreco-RomanClubHerning Brydeklub (DEN)[1]Coached byJarek Pzyara (since 2005)[2]Szymon Kog...


