Quingestrone
| |||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

本條目存在以下問題,請協助改善本條目或在討論頁針對議題發表看法。 此條目需要补充更多来源。 (2018年3月17日)请协助補充多方面可靠来源以改善这篇条目,无法查证的内容可能會因為异议提出而被移除。致使用者:请搜索一下条目的标题(来源搜索:羅生門 (電影) — 网页、新闻、书籍、学术、图像),以检查网络上是否存在该主题的更多可靠来源(判定指引)。 �...

Pemandangan Kota Bandung dari Masjid Raya Bandung. Berikut ini adalah daftar gedung tertinggi di Kota Bandung. Bangunan tertinggi Menurut tinggi Berikut ini daftar bangunan tertinggi di Kota Bandung menurut tinggi bangunan, berdasarkan standar Emporis. Tanda ≈ menunjukkan perkiraan tinggi bangunan dan bukan tinggi yang pasti. Peringkat Nama Gedung Tinggi Lantai Tahun Catatan 1 Parahyangan Residences A ≈133 m 33 2015 [1] 2 M Square Apartment ≈128 m 32 2018 [2] 3 Newton Th...

Department in Occitania, France This article is about the French department. For other uses, see Aveyron (disambiguation). Department of France in OccitaniaAveyron Avairon (Occitan)Department of FranceFrom top down, left to right: Conques, prefecture building in Rodez, Castle of Belcastel, the river Aveyron in Villefranche-de-Rouergue and Peyre FlagCoat of armsLocation of Aveyron in FranceCoordinates: 44°15′N 02°42′E / 44.250°N 2.700°E / 44.250; 2.700Count...

Protul Chandra SorcarP. C. SorcarLahir(1913-02-23)23 Februari 1913Tangail, Benggala, India BritaniaMeninggal6 Januari 1971(1971-01-06) (umur 57)Asahikawa, Hokkaidō, JapanKebangsaanIndia BritaniaPekerjaanPesulapSuami/istriBasanti Devi Protul Chandra Sorcar (23 Februari 1913 – 6 Januari 1971) adalah seorang pesulap India.[1] Ia adalah seorang pesulap yang aktif secara internasional selama 1950an dan 1960an, melakukan pementasan Indrajal di hadapan para penonton lan...

Jihadist organization in the Syrian Civil War (2012–2017) Al-Nusra Frontجبهة النصرة لأهل الشامFlag of Jabhat al-Nusra(January 2012 – July 2016)Flag of Jabhat Fatah al-Sham(July 2016 – January 2017)LeadersAbu Mohammad al-Julani (top emir)[1] Abu Abdullah al-Shami (senior member) Ahmad Salama Mabruk † (senior member)Abu Hajer al-Homsi † (top military commander)[2]Abu Omar al-Turkistani † (top military commander)[3]...

Actress Gertrude ElliottGertrude Elliott, from a 1904 publicationBornMay Gertrude Dermott(1874-12-14)December 14, 1874Rockland, Maine, U.S.DiedDecember 24, 1950(1950-12-24) (aged 76)NationalityAmericanOther namesLady Forbes-RobertsonOccupationActorSpouse Johnston Forbes-Robertson (m. 1900–1937) his deathChildren4, including Maxine (Blossom) Miles, Diana Forbes-Robertson, and Jean Forbes-RobertsonRelativesJoanna Van Gyseghe...

2020 Portuguese Grand PrixRace detailsRace 15 of 15 races in the2020 Grand Prix motorcycle racing seasonDate22 November 2020Official nameGrande Prémio MEO de PortugalLocationAutódromo Internacional do AlgarvePortimão, Algarve, PortugalCoursePermanent racing facility4.592 km (2.853 mi)MotoGPPole positionRider Miguel Oliveira KTMTime 1:38.892 Fastest lapRider Miguel Oliveira KTMTime 1:39.855 on lap 9 PodiumFirst Miguel Oliveira KTMSecond Jack Miller DucatiThird Franco M...

Pour les articles homonymes, voir Keller et Rosenberg. Lili Keller-Rosenberg LeignelBiographieNaissance 15 septembre 1932 (91 ans)CroixNom de naissance Lili RosenbergNationalité françaiseActivités Écrivain, conférencierAutres informationsDistinctions Officier de l'ordre national du Mérite (2017)Officier de la Légion d'honneur (2020)Commandeur des Arts et des Lettres (2023)Œuvres principales Je suis encore làEt nous sommes revenus seulsJ'avais votre âgemodifier - modifier...

Constituency of the Indian parliament in Bihar Purvi ChamparanLok Sabha constituencyConstituency detailsCountryIndiaRegionEast IndiaStateBiharAssembly constituenciesHarsidhiGovindganjKesariaKalyanpurPipraMotihariEstablished2008ReservationNoneMember of Parliament18th Lok SabhaIncumbent Radha Mohan Singh PartyBharatiya Janata PartyAllianceNDAElected year2024 Purvi Champaran Lok Sabha constituency is one of the 40 Lok Sabha (parliamentary) constituencies in Bihar state in eastern India.[1 ...

النيل الوطنية للنقل النهريالنيل الوطنية للنقل النهريمعلومات عامةالبلد مصر التأسيس 1960النوع مقاولة الشكل القانوني مؤسسات مملوكة للدولة المقر الرئيسي جمهورية مصر العربيةموقع الويب nationalniletrans.com (العربية) المنظومة الاقتصاديةالشركة الأم جهاز الصناعات والخدمات البحرية لل...
Este artigo ou seção pode conter informações desatualizadas. Se tem conhecimento sobre o tema abordado, edite a página e inclua as informações mais recentes, citando fontes fiáveis e independentes. —Encontre fontes: ABW • CAPES • Google (N • L • A) Parte de uma série sobre aPandemia de COVID-19Scientifically accurate atomic model of the external structure of SARS-CoV-2. Each ball is an atom. SARS-CoV-2 (vírus)COVID-19...
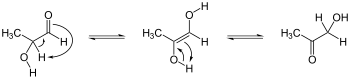
Organic compound with a C=C–OH group Examples of keto-enol tautomerismKetone tautomerization, keto-form at left, enol at right. Ex. is 3-pentanone, a less stabilized enol.[citation needed]Enolate resonance structures, schematic representation of forms (see text regarding molecular orbitals); carbanion form at left, enolate at right; Ex. is 2-butanone, also a less stabilized enol.[citation needed]Ketone tautomerization, enol-form at left, keto at right. Ex. is 2,4-pentanedion...
National highway in India National Highway 162AMap of the National Highway in redRoute informationLength50 km (31 mi)Major junctionsSouth endMavliNorth endKhandel LocationCountryIndiaStatesRajasthan Highway system Roads in India Expressways National State Asian ← NH 162→ NH 758 National Highway 162A, commonly called NH 162A is a national highway in India.[1][2] It is a spur road of National Highway 162.[3] NH-162A traverses the state of Raj...

此條目没有列出任何参考或来源。 (2023年5月4日)維基百科所有的內容都應該可供查證。请协助補充可靠来源以改善这篇条目。无法查证的內容可能會因為異議提出而被移除。 吴德施(Logan Herbert Roots,1870年—1945年)美国圣公会在华传教士,鄂湘教区主教。 1870年,吴德施出生在美国伊利诺斯州,1891年毕业于哈佛大学文学院,1896年毕业于圣公会神学院,同年由美国圣公会差会...

Mastos à figures noires. Vers 530 av. J.-C. Baltimore, Walters Art Museum, inv. 48.223. Un mastos (du grec ancien : μαστός, « sein ») est une forme de la céramique grecque antique, désignant un vase à boire dont l'apparence fait penser à un sein de femme. Le nom était déjà usité par les Grecs. Selon Athénée de Naucratis[1], « Apollodore de Cyrène, au dire de Pamphile, dit que les Paphiens appelaient ainsi le vase à boire ». Le mastos est en gén...

Tournoi Clausura2017 Généralités Sport Football Organisateur(s) FESFUT Édition 38e Date du 14 janvier 2017au 21 mai 2017 Participants 12 équipes Matchs joués 95 Site web officiel Site officiel Hiérarchie Hiérarchie 1er échelon Niveau inférieur Segunda División Palmarès Tenant du titre Santa Tecla FC Vainqueur Santa Tecla FC Deuxième Alianza FC Relégué(s) CD Universidad de El Salvador Navigation Saison précédente Saison suivante modifier Le Tournoi Clausura 2017 est le t...

French mathematician (1869–1951) Élie CartanProfessor Élie Joseph CartanBorn(1869-04-09)9 April 1869Dolomieu, Isère, FranceDied6 May 1951(1951-05-06) (aged 82)Paris, FranceAlma materUniversity of ParisKnown forLie groups (Cartan's theorem) Vector spaces and exterior algebra Differential geometry Special and general relativity Differential forms Quantum mechanics (spinors, rotating vectors) List of things named after Élie CartanChildrenHenri CartanRelativesAnna Cartan (sis...

Optical phenomenon Unpolarized light is light with a random, time-varying polarization. Natural light, like most other common sources of visible light, is produced independently by a large number of atoms or molecules whose emissions are uncorrelated. Unpolarized light can be produced from the incoherent combination of vertical and horizontal linearly polarized light, or right- and left-handed circularly polarized light.[1] Conversely, the two constituent linearly polarized states of ...

19th-century German writer Gustav FreytagGustav Freytag, from Die Gartenlaube (1886)Born13 July 1816Kreuzburg, Kingdom of PrussiaDied30 April 1895Wiesbaden, GermanyOccupationNovelist, playwrightSignature Gustav Freytag (German: [ˈfʁaɪˌtaːk]; 13 July 1816 – 30 April 1895) was a German novelist and playwright. Life Freytag was born in Kreuzburg (Kluczbork) in Silesia. After attending the school at Oels (Oleśnica), he studied philology at the universities of Breslau (Wrocław...

لايونز الإحداثيات 43°03′35″N 76°59′33″W / 43.0597°N 76.9925°W / 43.0597; -76.9925 [1] تقسيم إداري البلد الولايات المتحدة[2] التقسيم الأعلى مقاطعة وين عاصمة لـ مقاطعة وين خصائص جغرافية المساحة 97400000 متر مربع ارتفاع 137 متر عدد السكان عدد ال...
