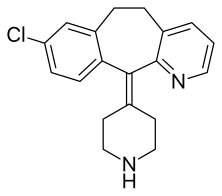
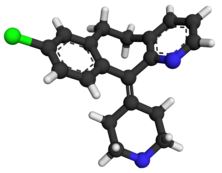
Desloratadine
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Artikel ini tentang tahun 1925. 1925MileniumMilenium ke-2AbadAbad ke-19Abad ke-20 Abad ke-21Dasawarsa 1900-an1910-an1920-an1930-an1940-anTahun1922192319241925192619271928 1925 (MCMXXV) merupakan tahun biasa yang diawali hari Kamis dalam kalender Gregorian, tahun ke-1925 dalam sebutan Masehi (CE) dan Anno Domini (AD), tahun ke-925 pada Milenium ke-2, tahun ke-25 pada Abad ke-20, dan tahun ke- 6 pada dekade 1920-an. Denominasi 1925 untuk tahun ini telah digunakan sejak periode Abad Perteng...

Thoroughfare in Toronto, Ontario For the avenue in Delaware County, Pennsylvania, see State Route 2005 (Delaware County, Pennsylvania). Lansdowne AvenueLansdowne Avenue in TorontoLooking north along Lansdowne AvenueRoute informationMaintained by City of Toronto governmentMajor junctionsSouth endQueen StreetMajor intersectionsDundas StreetBloor StreetDupont StreetNorth endSt. Clair Avenue LocationCountryCanadaProvinceOntario Highway system Roads in Toronto North–South East–West D...

العلاقات السعودية الجنوب سودانية السعودية جنوب السودان السعودية جنوب السودان تعديل مصدري - تعديل العلاقات السعودية الجنوب سودانية هي العلاقات الثنائية التي تجمع بين السعودية وجنوب السودان.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عام...

American baseball pitcher (born 1987) Baseball player Richard BleierBleier pitching for the Orioles in 2019Washington Nationals PitcherBorn: (1987-04-16) April 16, 1987 (age 36)Davie, Florida, U.S.Bats: LeftThrows: LeftMLB debutMay 30, 2016, for the New York YankeesMLB statistics (through 2023 season)Win–loss record15–6Earned run average3.27Strikeouts187 Teams New York Yankees (2016) Baltimore Orioles (2017–2020) Miami Marlins (2020–2022) Boston Red Sox (2023) Rich...

Синелобый амазон Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:ЗавропсидыКласс:Пт�...

Revised version of the Macintosh 128K by Apple Computer Not to be confused with Macintosh 512Ke. This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Macintosh 512K – news · newspapers · books · scholar · JSTOR (March 2023) (Learn how and when to remove this template message) Macintosh 512KAlso known asM0001WManu...

Major League Baseball season Major League Baseball team season 1905 Boston AmericansLeagueAmerican LeagueBallparkHuntington Avenue GroundsCityBoston, MassachusettsRecord78–74 (.513)League place4th (16 GB)OwnersJohn I. TaylorManagersJimmy CollinsStatsESPN.comBB-reference← 19041906 → Boston Americans manager Jimmy Collins The 1905 Boston Americans season was the fifth season for the professional baseball franchise that later became known as the Boston Red Sox. The A...

Pour les articles homonymes, voir Trente-et-Un-Octobre. Éphémérides Octobre 1er 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 31 septembre 31 novembre Chronologies thématiques Croisades Ferroviaires Sports Disney Anarchisme Catholicisme Abréviations / Voir aussi (° 1852) = né en 1852 († 1885) = mort en 1885 a.s. = calendrier julien n.s. = calendrier grégorien Calendrier Calendrier perpétuel Liste de calendriers Naissa...

Halaman ini berisi artikel tentang waralaba media. Untuk permainan pertama dalam waralaba ini, lihat Resident Evil (permainan video). Untuk other uses, lihat Resident Evil (disambiguasi). Resident EvilGenreSurvival horrorThird-person shooterFirst-person shooterPengembangCapcomPenerbitCapcomPembuat Shinji Mikami Tokuro Fujiwara Platform Daftar Arcade Dreamcast Game Boy Color GameCube iOS Microsoft Windows Mobile phones N-Gage Nintendo 3DS Nintendo 64 Nintendo DS Nintendo Switch PlayStation Pla...

Period. End of Sentence.Poster filmSutradaraRayka ZehtabchiProduserMelissa BertonGarrett SchiffSinematograferSam DavisPenyuntingSam DavisDistributorNetflixTanggal rilis 05 April 2018 (2018-04-05) (Cleveland International Film Festival) 19 Februari 2019 (2019-02-19) (Amerika Serikat) Durasi25 menitNegaraAmerika SerikatBahasaHindi Period. End of Sentence. adalah film dokumenter pendek tahun 2018 yang disutradarai oleh Rayka Zehtabchi.[1][2] Film ini memaparkan ...

Port Vale 1920–21 football seasonPort Vale1920–21 seasonChairmanFrank HuntbachManagerJoe SchofieldStadiumThe Old Recreation GroundFootball League Second Division17th (36 Points)FA CupSixth Qualification Round(knocked out by Clapton Orient)North Staffordshire Infirmary CupRunners-up(knocked out by Stoke)Top goalscorerLeague: Bobby Blood (20)All: Bobby Blood (20)Highest home attendance20,000 vs Rotherham County, 11 September 1920Stoke, 25 September 1920Lowest home attendance10,000 vs Wolve...

Deep-fried Iranian almond pastry QottabTypePastryPlace of origin IranRegion or stateYazdMain ingredientsFlour, almonds, powdered sugar, vegetable oil, cardamom Media: Qottab Qottab (Persian: قطاب qottâb) is an almond-filled deep-fried Iranian cuisine pastry or cake,[1] prepared with flour, almonds, powdered sugar, vegetable oil, and cardamom. The city of Yazd is well known for its qottab. Qottab developed from an earlier savoury pastry known as sanbosag, the ancest...

Сельское поселение России (МО 2-го уровня)Новотитаровское сельское поселение Флаг[d] Герб 45°14′09″ с. ш. 38°58′16″ в. д.HGЯO Страна Россия Субъект РФ Краснодарский край Район Динской Включает 4 населённых пункта Адм. центр Новотитаровская Глава сельского пос�...

Ideas from Mathematics have been used as inspiration for fiber arts A Möbius strip scarf made from crochet. Ideas from mathematics have been used as inspiration for fiber arts including quilt making, knitting, cross-stitch, crochet, embroidery and weaving. A wide range of mathematical concepts have been used as inspiration including topology, graph theory, number theory and algebra. Some techniques such as counted-thread embroidery are naturally geometrical; other kinds of textile provide a ...

French judicial school ENMÉcole nationale de la MagistratureTypePublicEstablished1959DirectorNathalie Roret[1]Academic staff100Students550LocationBordeaux, FranceWebsite[1] The French National School for the Judiciary (French: École nationale de la magistrature or ENM) is a French grande école, founded in 1958[2] by French President Charles de Gaulle and the father of the current French Constitution, Michel Debré, in order to encourage law students to embrace a judicial ca...
خوفوتمثال لخوفو بالمتحف المصريفرعون مصرسبقهسنفروتبعهچدف رع الألقاب الملكية اسم التتويج: خوفوḪw(j).f w(j)أنا محمي / هو يحميني[1] تهجئة بديلة: خنوم خوفو ẖnmw ḫwj=f w(j) خنوم يحميني اسم حورس: ميجيدو Mḏd.w[2]من يحطم أعداء حور تهجئة بديلة: الاسم النبتي: نبتي إر مجدد Mḏd-r-Nnb.tj...

Carlo Sbordone (Napoli, 23 novembre 1948) è un matematico italiano. Indice 1 Biografia 2 Attività scientifica 3 Principali risultati 4 Opere 5 Note 6 Collegamenti esterni Biografia Consegue la laurea in matematica nel 1970 presso l'Università degli Studi di Napoli Federico II, dove attualmente è professore emerito di Analisi matematica. È socio dell'Accademia Nazionale dei Lincei e presidente dell'Accademia Pontaniana. Attività scientifica Le ricerche di Carlo Sbordone riguardano princi...

Aircraft propulsion component This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Propeller aeronautics – news · newspapers · books · scholar · JSTOR (September 2011) (Learn how and when to remove this message) The propellers of a C-130J Super Hercules military transport aircraft In aeronautics, an aircraft...

American football player and coach (born 1951) For other people named John Mitchell, see John Mitchell (disambiguation). American football player John MitchellMitchell in Pittsburgh's Super Bowl XLIII paradePersonal informationBorn: (1951-10-14) October 14, 1951 (age 72)Mobile, Alabama, U.S.Height:6 ft 3 in (1.91 m)Weight:230 lb (104 kg)Career informationHigh school:Williamson (Mobile, Alabama)College:AlabamaEastern ArizonaNFL draft:1973 / Round: 7 ...

Russian table tennis player Alexey SmirnovPersonal informationFull nameAlexey SmirnovNationality RussiaBorn (1977-10-09) October 9, 1977 (age 46)Tolyatti, USSRHeight1.79 m (5 ft 10 in)Weight69 kg (152 lb) Medal record Men's table tennis Representing Russia European Championships 2003 Courmayeur Doubles 2005 Aarhus Doubles 2005 Aarhus Mixed Doubles 2007 Belgrade Doubles 2007 Belgrade Mixed Doubles Alexey Smirnov (born October 9, 1977) is a male table te...