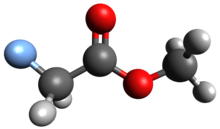
Methyl fluoroacetate
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Imam Al-Haramain adalah salah seorang ulama fikih, ahli ushul fikih, ilmuwan, agamawan, pemuka masyarakat, dan teolog muslim yang sering kali membahas persoalan-persoalan teologis secara mendalam, seperti persoalan fungsi akal dan wahyu, surga dan neraka, perbuatan manusia, dan lain-lain.[1][2] Dia dikenal sebagai pengikut aliran Sunni, dan uniknya, dalam komentar-komentarnya justru mengacu juga pada pemikiran-pemikiran Mu'tazilah.[1] Karena itulah dia disebut tokoh ko...

American politician George Dewey Clyde10th Governor of UtahIn officeJanuary 7, 1957 – January 4, 1965Preceded byJ. Bracken LeeSucceeded byCal Rampton Personal detailsBorn(1898-07-21)July 21, 1898Springville, UtahDiedApril 2, 1972(1972-04-02) (aged 73)Political partyRepublicanSpouseOra PackardChildren5Alma materB.S. Utah State University M.S. University of CaliforniaProfessionPolitician George Dewey Clyde (July 21, 1898 – April 2, 1972) was an American politician and the ...

Application in medieval astrology Astrology Background Worship of heavenly bodies History of astrology Astrology and astronomy Planets Behenian Classical Traditions, types, and systems Astrology and science Astrologers Astrological organizations Traditions Babylonian Chinese Hellenistic Hindu Islamic Jewish Tibetan Western Branches Natal Electional Horary Medical Financial Locational Psychological Meteorological Astrological signs Aries Taurus Gemini Cancer Leo Virgo Libra Scorpio Sagittarius...

Song by R.E.M Bittersweet MeSingle by R.E.M.from the album New Adventures in Hi-Fi B-sideUndertow (Live)ReleasedOctober 21, 1996 (1996-10-21)RecordedNovember 7, 1995StudioMemphis soundcheckLength4:06LabelWarner Bros.Songwriter(s)Bill BerryPeter BuckMike MillsMichael StipeProducer(s)Scott LittR.E.M.R.E.M. singles chronology E-Bow the Letter (1996) Bittersweet Me (1996) Electrolite (1997) Bittersweet Me is a song by American rock band R.E.M., released as the second single from th...

Синелобый амазон Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:ЗавропсидыКласс:Пт�...

2021 song by Roxen AmnesiaSingle by RoxenReleased4 March 2021Length2:54LabelWarnerSongwriter(s)Adelina StîngăVictor BouroșuProducer(s)Victor BouroșuRoxen singles chronology Parte din tine (2021) Amnesia (2021) Inimă nu fi de piatră (2021) Alternative coversInitial cover artwork Music videoAmnesia on YouTubeEurovision Song Contest 2021 entryCountryRomaniaArtist(s)RoxenComposer(s)Adelina StîngăVictor BouroșuLyricist(s)StîngăBouroșuFinals performanceSemi-final result12thSemi-final po...

† Человек прямоходящий Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:Синапсиды�...
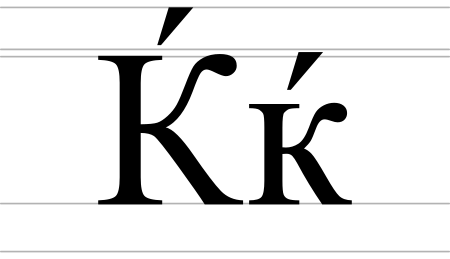
Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Kje Kiril – berita · surat kabar · buku · cendekiawan · JSTOR Huruf Kiril Kje (Tje) Penggunaan Fonetis:[c], [tɕ]Alfabet KirilHuruf SlaviaАА́А̀А̂А̄ӒБВГҐДЂЃЕЕ́ÈЕ̂ЁЄЖЗЗ́Ѕ...

Clarence Leonard Johnson Clarence Leonard Johnson, detto Kelly (Ishpeming, 27 febbraio 1910 – Los Angeles, 21 dicembre 1990), è stato un ingegnere statunitense. Indice 1 Biografia 2 Onorificenze 3 Bibliografia 4 Altri progetti 5 Collegamenti esterni Biografia Johnson entrò alla Lockheed nel 1933, forte di una laurea in ingegneria aeronautica all'università del Michigan. Prima dei trent'anni era già ingegnere capo della ditta, con al suo attivo il Lockheed L-10 Electra, il caccia P-38 Li...
Sakari Tuomioja Perdana Menteri FinlandiaMasa jabatan17 November 1953 – 5 Mei 1954PendahuluUrho KekkonenPenggantiRalf Törngren Informasi pribadiLahir29 Agustus 1911TampereMeninggal9 September 1964(1964-09-09) (umur 53)Partai politikNational Progressive PartySunting kotak info • L • B Sakari Severi Tuomioja (29 Agustus 1911 — 9 September 1964) adalah seorang politikus Finlandia (Partai Progresif Nasional), diplomat, Perdana Menteri Finlandia pada pemerintahan s...

Aleksandrs Solovjovs Nazionalità Lettonia Altezza 178 cm Peso 75 kg Calcio Ruolo Difensore, centrocampista Squadra Spartaks Jūrmala CarrieraGiovanili SkontoSquadre di club1 2005-2006 Skonto1 (0)2007→ Olimps Rīga24 (3)2008 Ventspils2 (0)2009→ Tranzit11 (0)2009-2010 Ventspils16 (3)2010-2011 Atromītos Geroskīpou5 (0)2011-2012 Nantwich Town18 (0)2012 Tauras15 (2)2013 Spartaks Jūrmala5 (0)2013-2014 Daugava42 (1)2015&#...

Artikel ini tentang penulis Injil Lukas dalam Alkitab. Untuk kitab di dalam Alkitab, lihat pula Injil Lukas SantoLukas PenginjilDetail altar St. Lukas, karya Andrea MantegnaRasul, PenginjilLahirAntiokia, Suriah, Kekaisaran RomawiMeninggalc. 84dekat Boiotia, YunaniDihormati diGereja Katolik Roma, Gereja Ortodoks Timur, Gereja Ortodoks Oriental, Gereja Katolik Timur, Gereja Anglikan, Gereja Lutheran, beberapa Protestan lainnyaTempat ziarahPadua, ItaliaPesta18 OktoberAtributPenginjil, dokter, us...
Programs by Voice of Vietnam This article may require cleanup to meet Wikipedia's quality standards. The specific problem is: Urls in references need repair. Please help improve this article if you can. (May 2022) (Learn how and when to remove this message) This is a list of programs that have been and are being broadcast by VTC Digital Television, belonging to the Voice of Vietnam. VTC1 Khoảnh khắc Vàng (tiền thân của Thời sự Tổng hợp) Sao Online Gia đình Online Khúc há...

Motor vehicle Simca 1100OverviewManufacturerSimcaAlso calledSimca 1200Simca 1118/1204 (US)Simca VFTalbot 1100Talbot 1200Dodge 1100 [1]Production1967–1985AssemblyPoissy, FranceMadrid, Spain (as Simca 1200)Nyköping, Sweden (Philipsons)[2]Body and chassisClassC-segment compact family carBody style3-door hatchback5-door hatchback5-door estate2-door coupe utility (pickup)3-door van3-door van (high roof)LayoutFront engine, front-wheel driveRelatedMatra RanchoPowertrain...

Brazilian racing driver (born 1952) This article is about the former Formula One World Champion. For his son, also a racing driver, see Nelson Piquet Jr. For other uses, see Nelson Piquet (disambiguation). In this Portuguese name, the first or maternal family name is Piquet and the second or paternal family name is Souto Maior. Nelson PiquetPiquet in 2022BornNelson Piquet Souto Maior (1952-08-17) 17 August 1952 (age 71)Rio de Janeiro, BrazilFormula One World Championship careerNation...

この記事は検証可能な参考文献や出典が全く示されていないか、不十分です。 出典を追加して記事の信頼性向上にご協力ください。(このテンプレートの使い方)出典検索?: 地下駅 – ニュース · 書籍 · スカラー · CiNii · J-STAGE · NDL · dlib.jp · ジャパンサーチ · TWL (2012年7月) 日本初の本格的な地下駅である宮城電気鉄道・仙台�...

French geographer (1837–1916) Onésime Reclus (1837–1916) Onésime Reclus (22 September 1837[1] – 30 June 1916) was a French geographer who specialized in the relations between France and its colonies. In 1880 he coined the term Francophonie as a means of classification of peoples of the world, being determined by the language they all spoke.[2] While this term did not appear in dictionaries until 1930, it has become more important since the late 20th century as part of ...

Territorial entity belonging to multiple nations This article is missing information about cross-border regions outside Europe. Please expand the article to include this information. Further details may exist on the talk page. (March 2020) A cross-border region is a territorial entity that is made of several local or regional authorities that are co-located yet belong to different nation states. Cross-border regions exist to take advantage of geographical conditions to strengthen their compet...

Type of bass lute from the 1700s This article is about a bass lute after 18th century. For a treble lute since 17th century or before, see Mandore (instrument). For other uses, see Mandora (disambiguation). Mandora or Gallichon Mandora (1726) 6~9 courses lute (Calchedon, Calichon) (1735)[1][2] Gallichon The mandora or gallichon is a type of 18th- and early 19th-century lute, with six to nine courses of strings. The terms were interchangeable, with mandora more commonly used fr...

この記事は検証可能な参考文献や出典が全く示されていないか、不十分です。 出典を追加して記事の信頼性向上にご協力ください。(このテンプレートの使い方)出典検索?: 矢崎化工 – ニュース · 書籍 · スカラー · CiNii · J-STAGE · NDL · dlib.jp · ジャパンサーチ · TWL (2020年7月) 矢崎化工株式会社Yazaki Kako Corporation. 本社種類 株�...