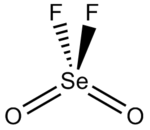
Selenoyl fluoride
| |||||||||||||||||||||||||||||||||||||||||
Read other articles:

Dwarka Sector 21द्वारका सेक्टर २१Stasiun angkutan cepat di DelhiJalur Jalur Biru Jalur Ekspres BandaraKonstruksiJenis strukturBawah TanahSejarahDibuka30 Oktober 2010 (Jalur Biru)23 Februari 2011 (Jalur Ekspres Bandara)Operasi layanan Stasiun sebelumnya Delhi Metro Stasiun berikutnya Dwarka Sector 8menuju Noida City Centre Jalur BiruTerminus Airportmenuju New Delhi Jalur JinggaTerminus Sunting kotak info • L • BBantuan pengg...

Australian politician The HonourableJohn MickelJohn Mickel speaks at a book launch in Brisbane, Australia in June 2011Speaker of the Legislative Assembly of QueenslandIn office21 April 2009 – 14 May 2012Preceded byMike ReynoldsSucceeded byFiona SimpsonMinister for Environment of QueenslandIn office12 February 2004 – 25 August 2004PremierPeter BeattiePreceded byDean WellsSucceeded byDesley Boyle (as Environment, Local Government, Planning and Women)Minister for Energy of ...

American ice hockey goaltender Ice hockey player Casey DeSmith DeSmith with the Pittsburgh Penguins in 2017Born (1991-08-13) August 13, 1991 (age 32)Rochester, New Hampshire, U.S.Height 6 ft 0 in (183 cm)Weight 181 lb (82 kg; 12 st 13 lb)Position GoaltenderCatches LeftNHL teamFormer teams Vancouver CanucksPittsburgh PenguinsNational team United StatesNHL Draft UndraftedPlaying career 2015–present Casey DeSmith (born August 13, 1991) is an Am...

German curler Peder LedosquetCurler ♂TeamCurling clubEC Oberstdorf, OberstdorfCurling career Member Association GermanyWorld Championshipappearances2 (1971, 1972) Medal record Curling World Championships 1972 Garmisch-Partenkirchen Peder Ledosquet is a former German curler. He is a 1972 World Men's bronze medallist.[1][2] Teams Season Skip Third Second Lead Events 1970–71 Manfred Räderer Peter Jacoby Peder Ledosquet Hansjörg Jacoby WCC 1971 (6th) 1971–72 ...

1811 novella by Friedrich de la Motte Fouqué Undine Cover of Undine, Told to the Children by Mary Macgregor, illustrated by Katharine Cameron (London: T. C. & E. C. Jack)AuthorFriedrich de la Motte FouquéCountryGermanyLanguageGermanGenreFantasy novellaPublication date1811Media typeMagazine story (later as a hardcover book) Undine is a fairytale novella (Erzählung) by Friedrich de la Motte Fouqué in which Undine, a water spirit, marries a knight named Huldbrand in order to gain a ...

Aminat Yusuf JamalAminat Yusuf Jamal pada tahun 2016Informasi pribadiKewarganegaraanBahrainLahir27 Juni 1997 (umur 26) OlahragaNegaraBahrainOlahragaAtletikLombaLari gawang 400 m Rekam medali Kejuaraan Dunia 2019 Doha Relay campuran Aminat Yusuf Jamal (lahir 27 Juni 1997) adalah seorang atlet Bahrain kelahiran Nigeria[1] yang mengkhususkan diri dalam lari gawang 400 meter.[2] Ia mewakili negara angkatnya pada Kejuaraan Dunia 2017 tanpa mencapai semi-final. Setahun sebelumn...

ХристианствоБиблия Ветхий Завет Новый Завет Евангелие Десять заповедей Нагорная проповедь Апокрифы Бог, Троица Бог Отец Иисус Христос Святой Дух История христианства Апостолы Хронология христианства Раннее христианство Гностическое христианство Вселенские соборы Н...

United Nations resolution adopted in 2002 UN Security CouncilResolution 1438Memorial to the Bali bombingsDate14 October 2002Meeting no.4,624CodeS/RES/1438 (Document)SubjectThreats to international peace and security caused by terrorist actsVoting summary15 voted forNone voted againstNone abstainedResultAdoptedSecurity Council compositionPermanent members China France Russia United Kingdom United StatesNon-permanent members Bulgaria Cameroon Colomb...

You can help expand this article with text translated from the corresponding article in French. (December 2016) Click [show] for important translation instructions. View a machine-translated version of the French article. Machine translation, like DeepL or Google Translate, is a useful starting point for translations, but translators must revise errors as necessary and confirm that the translation is accurate, rather than simply copy-pasting machine-translated text into the English Wikip...

تحتاج هذه المقالة إلى الاستشهاد بمصادر إضافية لتحسين وثوقيتها. فضلاً ساهم في تطوير هذه المقالة بإضافة استشهادات من مصادر موثوق بها. من الممكن التشكيك بالمعلومات غير المنسوبة إلى مصدر وإزالتها. بحاجة للاستشهاد بمعجم مطبوع بدلاً عن قاعدة بيانات معجمية على الإنترنت. م...

Військово-музичне управління Збройних сил України Тип військове формуванняЗасновано 1992Країна Україна Емблема управління Військово-музичне управління Збройних сил України — структурний підрозділ Генерального штабу Збройних сил України призначений для планува...

American industrialist, lawyer, and diplomat (1874–1962) This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Owen D. Young – news · newspapers · books · scholar · JSTOR (November 2022) (Learn how and when to remove this message) Owen YoungYoung, 1928Born(1874-10-27)October 27, 1874Stark, New York, U.S.DiedJuly...

1998 Cannes Film FestivalOfficial poster of the 51st Cannes Film Festival[1]Opening filmPrimary ColorsClosing filmGodzillaLocationCannes, FranceFounded1946AwardsPalme d'Or (Mia aioniotitakai mia mera)[2]Hosted byIsabelle HuppertNo. of films22 (En Competition)[3]27 (Un Certain Regard)8 (Out of Competition)15 (Cinéfondation)14 (Short Film)Festival date13 May 1998 (1998-05-13) – 24 May 1998 (1998-05-24)Websitefestival-cannes.com/enCannes F...

هيئة البيعة السعودية هيئة البيعة السعودية تفاصيل الوكالة الحكومية البلد السعودية تأسست 2006 صلاحياتها تتبع المملكة العربية السعودية المركز الرياض ، السعودية الإدارة المدير التنفيذي شاغر تعديل مصدري - تعديل هيئة البيعة السعودية، هي هيئة سعودية تعنى باختيار المل...

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (أكتوبر 2019) تضمن المادة 12 من دستور جمهورية سنغافورة المساواة والحماية بين جميع الناس أمام القانون. يحدد القانون أيضًا أربعة تصنيفات ممنوعة: الدين والعرق والنسب ومحل ال...

Schizzo che illustra du Locle Camille du Locle (Orange, 16 luglio 1832 – Capri, 9 ottobre 1903) è stato un librettista, impresario teatrale e regista teatrale francese. È ricordato per aver messo in scena la prima dell'opera di Bizet Carmen (1875). Indice 1 Biografia 2 Note 3 Altri progetti 4 Collegamenti esterni Biografia Impresario teatrale, originario della Vaucluse, dal 1862 fu assistente del suocero Émile Perrin all'Opéra national de Paris, prima di trasferirsi nel 1870 all'Opéra-...

Government ministry of Bangladesh People's Republic of Bangladesh Ministry of Religious Affairsধর্ম বিষয়ক মন্ত্রণালয়Government Seal of BangladeshMinistry overviewFormed1971JurisdictionGovernment of BangladeshHeadquartersBangladesh SecretariatDhaka-1000, Bangladesh.Annual budget500 crore taka 59 million dollarsMinister responsibleMd Faridul Haq Khan, MinisterMinistry executiveKazi Enamul Hassan ndc, SecretaryWebsitemora.gov.bd The Ministry of Religio...

British author and colonial administrator Sir William Lee-Warner Sir William Lee-Warner GCSI (18 April 1846 – 18 January 1914) was a British author and colonial administrator in the Indian Civil Service. He was Chief Commissioner of Coorg in 1895.[1][2][3] In 1907 he headed the eponymous Lee Warner Committee that examined Indians receiving education in Britain. Early life and education Lee-Warner was born in Little Walsingham[4] into a prominent Norfolk famil...

Season of television series Law & Order: Special Victims Unit Season of television series Law & Order: Special Victims UnitSeason 13Season 13 U.S. DVD coverStarring Mariska Hargitay Danny Pino Kelli Giddish Richard Belzer Ice-T Dann Florek No. of episodes23ReleaseOriginal networkNBCOriginal releaseSeptember 21, 2011 (2011-09-21) –May 23, 2012 (2012-05-23)Season chronology← PreviousSeason 12 Next →Season 14 List of episodes The thirteenth season of Law &...

Instituto Brasileiro de Geografia e Estatística Organização Natureza jurídica Fundação pública[1] Missão Retratar o Brasil com informações necessárias ao conhecimento da sua realidade e ao exercício da cidadania.[2] Atribuições Produção, análise, pesquisa e disseminação de informações de natureza estatística — demográfica e socioeconômica,e geocientífica — geográfica, cartográfica, geodésica e ambiental.[1] Dependência Ministério do Planejamento e Orçamento...