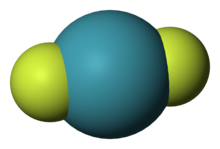
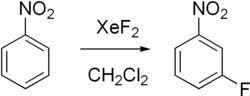
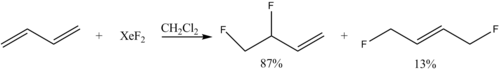
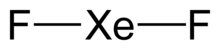Xenon difluoride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Lalat kepala tebal Conopidae Conops quadrifasciatusLalat mirip tawonTaksonomiKerajaanAnimaliaFilumArthropodaKelasInsectaOrdoDipteraUpaordoBrachyceraFamiliConopidae Latreille, 1802 Subfamili Conopinae Dalmanniinae Myopinae Notoconopinae Stylogastrinae lbs Conopidae atau lalat kepala tebal adalah famili lalat yang berada di subordo Brachycera di Diptera, dan menjadi satu-satunya anggota superfamili Conopoidea. Lalat-lalat dari famili ini dapat ditemukan diseluruh wilayah biogeografis dunia kecu...

Disambiguazione – Se stai cercando altri significati, vedi Provincia di Mantova (disambigua). Provincia di Mantovaprovincia Provincia di Mantova – VedutaPalazzo della Cervetta, sede della Provincia LocalizzazioneStato Italia Regione Lombardia AmministrazioneCapoluogoMantova PresidenteCarlo Bottani (lista civica di centro-destra) dal 18-12-2021[1] Data di istituzione1786 TerritorioCoordinatedel capoluogo45°10′N 10°48′E / 45.166667°N 10.8...

Mecha berfokus pada teknologi seperti robot yang di kendalikan manusia pada cerita fiksi Istilah mecha (Jepang: メカcode: ja is deprecated , Hepburn: meka) merujuk kepada gagasan ilmiah dan genre fiksi ilmiah yang terpusat pada robot atau mesin raksasa yang dikendalikan oleh manusia di dalamnya. Mecha biasanya digambarkan sebagai robot peranti bergerak humanoid. Istilah tersebut mula-mula dipakai dalam bahasa Jepang (meka) yang diambil dari padanan Inggrisnya mekanikaru ('mekanikal'), tetap...

Georges CouthonGeorges Auguste Couthon oleh Bonneville, Musée Carnavalet, Paris Anggoya Komite Keamanan MasyarakatMasa jabatan10 Juli 1793 – 28 Juli 1794Presiden Konvensi NasionalMasa jabatan21 Desember 1793 – 5 Januari 1794 PendahuluJean-Henri VoullandPenggantiJacques Louis DavidDeputi Konvensi NasionalMasa jabatan20 September 1792 – 10 Juli 1794 PenggantiGilbert-Amable JourdeDaerah pemilihanPuy-de-Dôme Informasi pribadiLahir(1755-12-22)22 Desember 1755Orce...

Artikel ini perlu diwikifikasi agar memenuhi standar kualitas Wikipedia. Anda dapat memberikan bantuan berupa penambahan pranala dalam, atau dengan merapikan tata letak dari artikel ini. Untuk keterangan lebih lanjut, klik [tampil] di bagian kanan. Mengganti markah HTML dengan markah wiki bila dimungkinkan. Tambahkan pranala wiki. Bila dirasa perlu, buatlah pautan ke artikel wiki lainnya dengan cara menambahkan [[ dan ]] pada kata yang bersangkutan (lihat WP:LINK untuk keterangan lebih lanjut...

Koleksi Spring 2007 dalam Pekan Mode London. Koleksi Alexander Wang dalam Pekan Mode New York 2010. Seorang peragawan dalam Pekan Mode Paris. Pekan busana atau pekan mode adalah sebuah pagelaran dalam industri busana, diselenggarakan selama satu minggu, dan menjadi ajang bagi para perancang busana, merek busana atau rumah busana untuk memeragakan koleksi terbaru mereka, dan para penikmat busana serta media juga berkesempatan untuk meliput dan menyaksikan tren busana terbaru. Ajang ini memungk...

County in Oklahoma, United States County in OklahomaMcClain CountyCountyMcClain County CourthouseLocation within the U.S. state of OklahomaOklahoma's location within the U.S.Coordinates: 35°00′N 97°26′W / 35°N 97.44°W / 35; -97.44Country United StatesState OklahomaFounded1907SeatPurcellLargest cityNewcastleArea • Total580 sq mi (1,500 km2) • Land571 sq mi (1,480 km2) • Water9.6 sq&...

Vittorio Sereni nel 1975 Vittorio Sereni (Luino, 27 luglio 1913 – Milano, 10 febbraio 1983) è stato un poeta, scrittore e traduttore italiano. Indice 1 Biografia 1.1 L'infanzia a Luino 1.2 Il trasferimento a Milano e i primi contatti con gli intellettuali 1.3 L'esperienza della guerra e la prigionia in Algeria 1.4 Il ritorno a Milano: il lavoro alla Pirelli e alla Mondadori 1.5 Gli ultimi anni, la morte e la memoria 2 Poetica 3 Opere 3.1 Poesia 3.1.1 Raccolte 3.1.2 Poesie in volume 3.1.3 R...

Синелобый амазон Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:ЗавропсидыКласс:Пт�...

Cet article est une ébauche concernant l’art. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Le Jugendstil (également appelé Art nouveau), équivalent en Allemagne de l'Art nouveau, est un mouvement artistique moderniste international embrassant toutes les disciplines à la fin du XIXe siècle. Les peuples germanophones, ou situés dans cette sphère d'influence, utilisèrent également à cette époq...

Virginia Slims of California 1986 Sport Tennis Data 24 febbraio - 2 marzo Edizione 16a Superficie Sintetico indoor Campioni Singolare Chris Evert-Lloyd Doppio Hana Mandlíková / Wendy Turnbull 1985 1987 Il Virginia Slims of California 1986 è stato un torneo di tennis giocato sul sintetico indoor. È stata la 16ª edizione del torneo, che fa parte del Virginia Slims World Championship Series 1986. Si è giocato a San Francisco negli Stati Uniti, dal 24 febbraio al 2 marzo 1986. Indice 1 Cam...

У этого термина существуют и другие значения, см. Чайки (значения). Чайки Доминиканская чайкаЗападная чайкаКалифорнийская чайкаМорская чайка Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:Вторич...

Map all coordinates using OpenStreetMap Download coordinates as: KML GPX (all coordinates) GPX (primary coordinates) GPX (secondary coordinates) Suburb of Brisbane, Queensland, AustraliaCannon HillBrisbane, QueenslandCannon Hill railway stationCannon HillCoordinates27°28′24″S 153°05′48″E / 27.4734°S 153.0968°E / -27.4734; 153.0968 (Cannon Hill (centre of suburb))Population6,701 (2021 census)[1] • Density1,763/km2 (4,570/sq&...

Zaō 蔵王町KotaprajaBalai Kota Zaō BenderaEmblemLokasi Zaō di Prefektur MiyagiZaōLokasi di JepangKoordinat: 38°05′53.1″N 140°39′31.2″E / 38.098083°N 140.658667°E / 38.098083; 140.658667Koordinat: 38°05′53.1″N 140°39′31.2″E / 38.098083°N 140.658667°E / 38.098083; 140.658667Negara JepangWilayahTōhokuPrefektur MiyagiDistrikKattaPemerintahan • WalikotaHideto MurakamiLuas • Total152,83&...

Codice d'onoreTom Cruise in una scena del filmTitolo originaleA Few Good Men Paese di produzioneStati Uniti d'America Anno1992 Durata138 min Generedrammatico RegiaRob Reiner SoggettoAaron Sorkin SceneggiaturaAaron Sorkin, William Goldman (non accreditato) ProduttoreDavid Brown, Rob Reiner, Andrew Scheinman Produttore esecutivoWilliam S. Gilmore, Rachel Pfeffer Casa di produzioneColumbia Pictures, Castle Rock Entertainment Distribuzione in italianoColumbia TriStar Films Italia FotografiaRobert...

British civil servant and writer (1932–2023) SirBernard InghamIngham in 1987Downing Street Press SecretaryIn office1979 – 28 November 1990Prime MinisterMargaret ThatcherPreceded byHenry JamesSucceeded byGus O'Donnell Personal detailsBorn(1932-06-21)21 June 1932Halifax, EnglandDied24 February 2023(2023-02-24) (aged 90)Caterham, EnglandSpouse Nancy Hoyle (m. 1956; died 2017)Children1EducationHebden Bridge Grammar SchoolBradfo...

Hungarian pianist, teacher, and composer For the Diebold whistleblower, see Premier Election Solutions § Stephen Heller (whistleblower). For other people with the same name, see Steve Heller (disambiguation). The native form of this personal name is Heller István. This article uses Western name order when mentioning individuals. Stephen Heller by Alfred Lemoine. Stephen Heller (15 May 1813 – 14 January 1888) was a Hungarian pianist, teacher, and composer whose career...

Political party in Hawaii Aloha ʻĀina Party Hawaiian: ʻAoʻao Aloha ʻĀinaChairpersonJoyclynn CostaFoundedJune 1, 2015; 9 years ago (2015-06-01)HeadquartersHonoluluIdeologyAloha ʻĀina Hawaiian sovereigntyColorsRed and yellowSeats in the Upper House0 / 25Seats in the Lower House0 / 51Websitewww.alohaainaparty.infoPolitics of the United StatesPolitical partiesElections The Aloha ʻĀina Party (Hawaiian for love of the land) is a political party in the US state of Hawaiʻ...

Manga Shirahime-Syo: Snow Goddess TalesCover of the English-language translation as published by Viz Media (2015)白姫抄(Shirahimeshō)GenreSupernatural MangaWritten byClampPublished byKobunshaKadokawa ShotenEnglish publisherNA: Tokyopop (former)Viz MediaDemographicShōjoPublishedJune 10, 1992Volumes1 Shirahime-Syo: Snow Goddess Tales (Japanese: 白姫抄, Hepburn: Shirahimeshō) is a supernatural manga by Clamp, a creative team made up by Satsuki Igarashi, Nanase Ohkawa, Tsubaki ...

Ilse Aichinger (Vienna, 1º novembre 1921 – Vienna, 11 novembre 2016) è stata una scrittrice austriaca. Indice 1 Vita 2 Opere 3 Premi e riconoscimenti 4 Note 5 Altri progetti 6 Collegamenti esterni Vita Ilse Aichinger[1] e la sorella gemella Helga nacquero a Vienna. Il padre era insegnante, la madre ebrea medico. Dopo la loro separazione nel 1926 Aichinger visse con la madre a Vienna. Nel periodo del nazismo[2] la madre perse il lavoro. La nonna e i fratelli minori della ma...