Krypton difluoride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Katedral Casale MonferratoKatedral Santo Evasius dan Santo LaurensiusItalia: Cattedrale di S. Evasio e S. Lorenzocode: it is deprecated Katedral Casale MonferratoLokasiCasale MonferratoNegaraItaliaDenominasiGereja Katolik RomaArsitekturStatusKatedralStatus fungsionalAktifAdministrasiKeuskupanKeuskupan Casale Monferrato Katedral Casale Monferrato (Italia: Duomo di Casale Monferrato; Cattedrale di Sant'Evasiocode: it is deprecated ) adalah gereja katedral Katolik yang terletak di Casale Monferr...

Senegalese footballer For other people named Mamadou Coulibaly, see Mamadou Coulibaly (disambiguation). Mamadou Coulibaly Coulibaly with Pescara in 2017Personal informationDate of birth (1999-02-03) 3 February 1999 (age 25)Place of birth Thiès, SenegalHeight 1.83 m (6 ft 0 in)Position(s) Defensive midfielderTeam informationCurrent team Palermo(on loan from Salernitana)Number 80Youth career2017 PescaraSenior career*Years Team Apps (Gls)2017 Pescara 9 (0)2017–2022 Udinese...

العلاقات الأندورية البولندية أندورا بولندا أندورا بولندا تعديل مصدري - تعديل العلاقات الأندورية البولندية هي العلاقات الثنائية التي تجمع بين أندورا وبولندا.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين: وجه المقارنة...

يفتقر محتوى هذه المقالة إلى الاستشهاد بمصادر. فضلاً، ساهم في تطوير هذه المقالة من خلال إضافة مصادر موثوق بها. أي معلومات غير موثقة يمكن التشكيك بها وإزالتها. (ديسمبر 2018) غواتيمالا في الألعاب الأولمبية علم غواتيمالا رمز ل.أ.د. GUA ل.أ.و. اللجنة الأولمبية الغواتيمالي�...

Sports stadium in Maumee, Ohio, United States Ned Skeldon StadiumStadium in 2019Former namesLucas County StadiumLocation2901 Key Street Maumee, Ohio 43537Coordinates41°35′04″N 83°38′39″W / 41.58456°N 83.644203°W / 41.58456; -83.644203OwnerLucas CountyCapacity10,197[citation needed]Field sizeLeft field: 325 ftCenter field: 410 ftRight field: 325 ft[citation needed]Opened1965Closed2022TenantsToledo Mud Hens (IL) (1965–2002) Game at the stadi...

American political campaign Marianne Williamson for PresidentCampaign2024 U.S. presidential election (Democratic Party primaries)CandidateMarianne WilliamsonAnnouncedMarch 4, 2023February 28, 2024HeadquartersWashington, D.C.Key peopleCarlos Cardona (former campaign manager)[1][2]Peter Daou (former campaign manager)[3]Harvey J. Kaye (campaign advisor)[4]Robin Vogt (national volunteer coordinator)[5][better source needed][6]...

French film director Valérie GuignabodetValérie Guignabodet in 2009Born(1965-05-09)9 May 1965Paris, FranceDied23 February 2016(2016-02-23) (aged 50)Saint-Andiol, FranceEducationEmlyon Business SchoolOccupation(s)Director, screenwriterYears active2002–2016 Valérie Guignabodet (9 May 1965 – 23 February 2016) was a French film director and screenwriter.[1] She studied at Emlyon Business School,[2] and directed four films between 2002 and 2009, including Danse...

Sceaux 行政国 フランス地域圏 (Région) イル=ド=フランス地域圏県 (département) オー=ド=セーヌ県郡 (arrondissement) アントニー郡小郡 (canton) 小郡庁所在地INSEEコード 92071郵便番号 92330市長(任期) フィリップ・ローラン(2008年-2014年)自治体間連合 (fr) メトロポール・デュ・グラン・パリ人口動態人口 19,679人(2007年)人口密度 5466人/km2住民の呼称 Scéens地理座標 北緯48度4...

「アプリケーション」はこの項目へ転送されています。英語の意味については「wikt:応用」、「wikt:application」をご覧ください。 この記事には複数の問題があります。改善やノートページでの議論にご協力ください。 出典がまったく示されていないか不十分です。内容に関する文献や情報源が必要です。(2018年4月) 古い情報を更新する必要があります。(2021年3月)出...

List of events ← 1900 1899 1898 1901 in the United States → 1902 1903 1904 Decades: 1880s 1890s 1900s 1910s 1920s See also: History of the United States (1865–1918) Timeline of United States history (1900–1929) List of years in the United States 1901 in the United States1901 in U.S. states and territories States Alabama Arkansas California Colorado Connecticut Delaware Florida Georgia Idaho Illinois Indiana Iowa Kansas Kentucky Louisiana Maine Maryland Massachusetts Michigan M...

National museum in Tripoli, LibyaRed Castle Museum also known as: • Assaraya Alhamra Museum (Arabic: متحف السرايا الحمراء) • Archaeological Museum of TripoliEstablished1919LocationTripoli, LibyaTypeNational museum The Red Castle and entrance to the national Red Castle Museum The Red Castle Museum, also known as As-saraya Al-hamra Museum (Arabic: متحف السرايا الحمراء), the Archaeological Museum of Tripoli or Jamahiriya Museu...

Chapter of the New Testament Luke 20← chapter 19chapter 21 →Facsimile from 1861 of Luke 20:9 in Codex Cyprius (9th-10th century).BookGospel of LukeCategoryGospelChristian Bible partNew TestamentOrder in the Christian part3 Luke 20 is the twentieth chapter of the Gospel of Luke in the New Testament of the Christian Bible. It records the teaching of Jesus Christ in the temple in Jerusalem, especially his responses to questions raised by the Pharisees and Sadducees.[1] The ...

Australian bass-baritone and songwriter (1882 - 1961) For other uses, see Peter Dawson. This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (February 2014) (Learn how and when to remove this message) Peter DawsonBackground informationBirth namePeter Smith DawsonAlso known asJ. P. McCall, Will Strong, Will Danby, Hector Grant, Arthur Walpole, Robert Woodville, Evel...
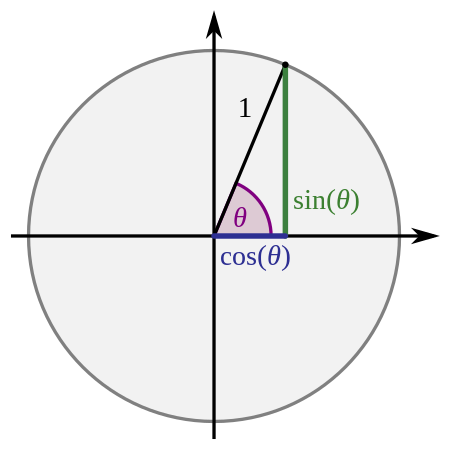
Fundamental trigonometric functions Sine and Cosine redirect here. For other uses, see Sine (disambiguation) and Cosine (disambiguation). Sine is not to be confused with Sign, Sign (mathematics) or the sign function. Sine and cosineGeneral informationGeneral definition sin ( α ) = opposite hypotenuse cos ( α ) = adjacent hypotenuse {\displaystyle {\begin{aligned}&\sin(\alpha )={\frac {\textrm {opposite}}{\textrm {hypotenuse}}}\\[8pt]&\cos(\alpha )={\fra...

International border Map of the Algeria-Western Sahara border The Algeria–Western Sahara border is 41 kilometres (25 mi) in length and runs from the tripoint with Morocco in the north to the tripoint with Mauritania in the south.[1][2] Description The border consists of relatively short north–south straight line running through the Sahara desert connecting the Moroccan and Mauritanian tripoints. History France occupied much of the northern coastal areas of Algeria in ...

Questa voce sugli argomenti stati scomparsi e Colonie è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Questa voce sull'argomento Delaware è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Colonia del Delaware Dati amministrativiNome completoDelaware Colony Lingue ufficialiInglese Lingue parlateInglese CapitaleNew Castle Dipendente da Regno d'Inghilterra (1664-1707...

I capi di governo della Polonia si sono avvicendati a partire dal 1917, col titolo di: presidente dei ministri (Prezydent Ministrów), fino al 1921, quando entrò in vigore la Costituzione di marzo; presidente del Consiglio dei ministri (Prezes Rady Ministrów) dal 1921 in poi. Indice 1 Lista 1.1 Regno di Polonia 1.2 Repubblica Popolare di Polonia 1.3 Seconda Repubblica di Polonia 1.4 Repubblica Popolare di Polonia (1944-1989) 1.5 Governi in esilio (1939-1990) 1.6 Repubblica di Polonia 2 Line...

Questa voce o sezione sull'argomento chimica è priva o carente di note e riferimenti bibliografici puntuali. Sebbene vi siano una bibliografia e/o dei collegamenti esterni, manca la contestualizzazione delle fonti con note a piè di pagina o altri riferimenti precisi che indichino puntualmente la provenienza delle informazioni. Puoi migliorare questa voce citando le fonti più precisamente. Segui i suggerimenti del progetto di riferimento. Idrossido di sodioidrossido di sodio cristallo...

This article may rely excessively on sources too closely associated with the subject, potentially preventing the article from being verifiable and neutral. Please help improve it by replacing them with more appropriate citations to reliable, independent, third-party sources. (September 2016) (Learn how and when to remove this message) The School of Health in Social Science at the University of Edinburgh is a department undertaking research and teaching into health, health policy and related ...

Flughafen Berlin und BER sind Weiterleitungen auf diesen Artikel. Zu historischen Flughäfen siehe die Liste der Berliner Flughäfen, zu weiteren Bedeutungen siehe Ber. Flughafen Berlin Brandenburg„Willy Brandt“ Luftbild des Flughafengeländes von Osten (2019) Berlin Brandenburg (Brandenburg) Berlin Brandenburg Kenndaten ICAO-Code EDDB IATA-Code BER Flugplatztyp Verkehrsflughafen Koordinaten 52° 21′ 44″ N, 13° 30′ 2″ O52.36224722222213.500672222222...


