Xenon tetrafluoride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

The following is a List of Bangladeshi inventions and discoveries which lists inventions and discoveries made by Bangladeshis both within Bangladesh and outside the region, which owe their existence either partially or entirely to a person born in Bangladesh. Inventions and improvements The following is a list of inventions, innovations or discoveries known or generally recognized to be Bangladeshi. Topic Inventions and discoveries Image Reference Science and medicine Sono arsenic filter was...

Domestique diarahkan ke halaman ini. Untuk istilah lain, lihat Domestik (disambiguasi). Domestique dari beberapa tim membentuk jalur di depan peloton untuk menjaga pemimpin mereka tetap didekat bagian depan balapan. Perhatikan George Hincapie bekerja untuk pemimpin timnya Lance Armstrong, terlihat dalam jaket kuning di Tour de France. Seorang domestique/Penolong adalah seorang pembalap sepeda jalan raya yang bekerja untuk kepentingan tim dan pimpinannya. Dalam bahasa Prancis, domestique diter...

Judah Loew ben BezalelPatung Loew karya Ladislav Šaloun di Balai Kota Baru Praha, Republik CekoPenjelasan pribadiLahirantara 1512 dan 1526Poznań, PolandiaWafat17 September 1609Praha, BohemiaDimakamkanPemakaman Yahudi Lama, PrahaTanda tangan Judah Loew ben Bezalel (Ibrani: יהודה ליווא בן בצלאלcode: he is deprecated ; antara 1512 dan 1526 – 17 September 1609), juga disingkat menjadi Rabi Loew (alternatif: Löw, Loewe, Löwe atau Levai), Maharal dari Praha (Ibrani: מהר״�...
Arnschwang Lambang kebesaranLetak Arnschwang NegaraJermanNegara bagianBayernWilayahOberpfalzKreisChamSubdivisions24 OrtsteilePemerintahan • MayorMichael Multerer (Bürgerblock) (CSU)Luas • Total28,30 km2 (1,090 sq mi)Ketinggian390 m (1,280 ft)Populasi (2013-12-31)[1] • Total1.940 • Kepadatan0,69/km2 (1,8/sq mi)Zona waktuWET/WMPET (UTC+1/+2)Kode pos93473Kode area telepon0 99 77Pelat kendaraanCHASitus web...
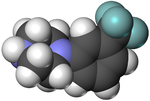
Chemical compound TrifluoromethylphenylpiperazineClinical dataRoutes ofadministrationOralATC codenoneLegal statusLegal status AU: S9 (Prohibited substance) BR: Class F2 (Prohibited psychotropics)[1] CA: Schedule III DE: Anlage II (Authorized trade only, not prescriptible) NZ: Class C US: Scheduled in Florida; Unscheduled Federally II-P (Poland)[2] Pharmacokinetic dataMetabolismLiverCYP2D6, CYP1A2, CYP3A4Identifiers IUPAC name 1-[3-(trifluoromethyl...

Об экономическом термине см. Первородный грех (экономика). ХристианствоБиблия Ветхий Завет Новый Завет Евангелие Десять заповедей Нагорная проповедь Апокрифы Бог, Троица Бог Отец Иисус Христос Святой Дух История христианства Апостолы Хронология христианства Ран�...

Federasi Sepak Bola KenyaCAFDidirikan1960Bergabung dengan FIFA1960Bergabung dengan CAF1968PresidenMohammed HatimyWebsitewww.kff.co.ke Federasi Sepak Bola Kenya (Inggris: Kenya Football Federation (KFF) adalah badan pengendali sepak bola di Kenya. Kompetisi Badan ini menyelenggarakan beberapa kompetisi di Kenya, yakni: Liga Utama Kenya Liga Nasional Kenya Piala Federasi Sepak Bola Kenya Tim nasional Badan ini juga merupakan badan pengendali dari 3 tim nasional di Kenya, yakni: Tim nasional...

Théodore VienneThéodore Vienne en 1913BiographieNaissance 28 juillet 1864RoubaixDécès 1er mars 1921 (à 56 ans)15e arrondissement de ParisSépulture Cimetière du Père-LachaiseNationalité françaiseActivités Homme d'affaires, mécène, journaliste, officiel du sportAutres informationsSports Sport cycliste, billard français, boxemodifier - modifier le code - modifier Wikidata Théodore Vienne, né à Roubaix le 29 juillet 1864 et mort à Paris le 1er mars 1921, est un industriel d...

Синелобый амазон Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:ЗавропсидыКласс:Пт�...

Museum Istana Kekaisaran Shenyang沈阳故宫Pemandangan dari atas dari Istana MukdenLocation within LiaoningTampilkan peta LiaoningIstana Mukden (Tiongkok)Tampilkan peta TiongkokDidirikan1955LokasiNo. 171, Shenyang Road, Distrik Shenhe, Shenyang, LiaoningKoordinat41°47′46″N 123°27′03″E / 41.796161°N 123.450708°E / 41.796161; 123.450708JenisMuseum seni, Istana Kekaisaran, situs bersejarahWisatawan1.6 juta[1]KuratorBin WuLuas6 hektar[2]Dibang...

Katedral CanterburyCathedral Canterbury Katedral Canterbury dari gerbang kota Informasi Lokasi Canterbury Nama lengkap Katedral dan Gereja Kristen Metropolit CanterburyCathedral and Metropolitical Church of Christ at Canterbury Koordinat geografi 51°16′47″N 1°04′59″E / 51.279722°N 1.083056°E / 51.279722; 1.083056Koordinat: 51°16′47″N 1°04′59″E / 51.279722°N 1.083056°E / 51.279722; 1.083056 Kaunti Kent Negara Britania Ray...

Zastava M91 Zastava M91 Jenis Senapan runduk Negara asal FR Yugoslavia Sejarah pemakaian Masa penggunaan 1992-sekarang Digunakan oleh Lihat Pengguna Pada perang Perang Yugoslavia,[1] Perang Kosovo Sejarah produksi Tahun 1991 Produsen Zastava Arms Jumlah produksi 4,000[butuh rujukan] Spesifikasi Berat 515 kg (1.135,4 pon) (dengan bidikan optik) Panjang 1.195 mm (47,05 in) Panjang laras 620 mm (24,41 in) Peluru 7...

Scottish journalist and television presenter (born 1955) Kirsty WarkFRSEWark in 2008BornKirsteen Anne Wark (1955-02-03) 3 February 1955 (age 69)Dumfries, ScotlandEducationWellington School, AyrAlma materUniversity of EdinburghOccupationTelevision journalistEmployerBBCNotable creditNewsnightSpouse Alan Clements (m. 1989)Children2 Kirsteen Anne Kirsty Wark FRSE (born 3 February 1955) is a Scottish television presenter with a long career at the BBC. Start...

Chemical compound Not to be confused with sodium nitrate, sodium nitride, or nitratine. E250 redirects here. For the mobile phone, see Samsung SGH-E250. Sodium nitrite The nitrite anion (space-filling model) The sodium cation Unit cell of sodium nitrite under standard conditions Names IUPAC name Sodium nitrite Identifiers CAS Number 7632-00-0 Y 3D model (JSmol) Interactive image ChEBI CHEBI:78870 N ChEMBL ChEMBL93268 Y ChemSpider 22689 Y ECHA InfoCard 100.028.687 EC Number...

Disambiguazione – Se stai cercando altri significati, vedi Ambrosino (disambigua). Ambrosino (in latino ambroxsinus),[1] anche chiamato ambrogino o ambrogiano,[2] è il nome delle monete coniate a Milano nel medioevo raffiguranti Sant'Ambrogio. Indice 1 Storia 1.1 Premesse 1.2 Ambrosino grosso 1.3 Ambrosino piccolo 1.4 Ambrosino grandissimo 1.5 L'ambrosino d'oro 2 Monete 3 Note 4 Bibliografia 5 Voci correlate 6 Altri progetti Storia Premesse Grosso da 6 denari imperial...

68th season of the ARCA Menards Series West 2021 ARCA Menards Series West Previous 2020 Next 2022 Champions | Seasons Jesse Love, the 2021 ARCA Menards Series West champion. Jake Drew lost the tiebreaker over Love. Cole Moore finished third in the final standings. The 2021 ARCA Menards Series West was the sixty-eighth season of the ARCA Menards Series West, a regional stock car racing series sanctioned by NASCAR in the United States. The season began at Phoenix Raceway with the Gene...

Kualifikasi untuk kelolosan (UEFA) Piala Dunia FIFA 1958 1962 1966 1970 1974 1978 1982 1986 1990 1994 1998 2002 2006 2010 2014 2018 Artikel utama: Kualifikasi Piala Dunia FIFA Piala Eropa 1960 1964 1968 1972 1976 1980 1984 1988 1992 1996 2000 2004 2008 2012 2016 2020 Artikel utama: Kualifikasi Kejuaraan Eropa UEFA lbs Babak kualifikasi untuk Piala Eropa 1976 diikuti oleh 32 tim yang dibagi menjadi delapan grup yang berisi empat tim. Pemenang dari setiap grup lolos ke perempat-final. Babak per...
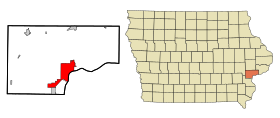
موسكاتاين الإحداثيات 41°25′26″N 91°03′22″W / 41.423888888889°N 91.056111111111°W / 41.423888888889; -91.056111111111 [1] تاريخ التأسيس 1839 تقسيم إداري البلد الولايات المتحدة[2][3] التقسيم الأعلى مقاطعة موسكاتاين عاصمة لـ مقاطعة موسكاتاين خصائص جغرافية ا�...

الجنية القرصانةThe Pirate Fairy (بالإنجليزية) ملصق الفيلممعلومات عامةالتصنيف فيلم رسوم متحركة الصنف الفني فنتازيا رسوم متحركة مغامراتتاريخ الصدور 13 فبراير 2014 الدنمارك 1 أبريل 2014 الولايات المتحدةمدة العرض 78 دقيقةاللغة الأصلية الإنجليزيةالبلد الولايات المتحدةموقع الويب fairies...

Dutch Golden Age portrait painter David van der PlasDavid van der Plas and Maria Sybille Merian in Arnold Houbraken's Schouwburg.Born(1647-12-11)11 December 1647AmsterdamDied18 May 1704(1704-05-18) (aged 56)AmsterdamNationalityDutchKnown forPaintingMovementDutch Golden Age painting David van der Plas (11 December 1647 – 18 May 1704), was a Dutch Golden Age portrait painter. Biography Van der Plas' portrait of Cornelis Tromp Dortsman's Round Lutheran church in Amsterdam Doll ho...



