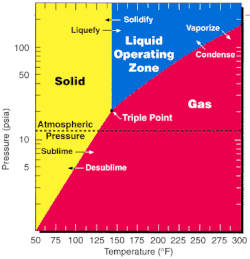
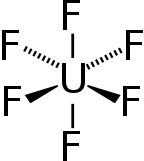
Uranium hexafluoride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Gandum Triticum aestivum, jenis gandum yang paling umum ditanam. Klasifikasi ilmiah Kerajaan: Plantae Divisi: Magnoliophyta Kelas: Liliopsida Ordo: Poales Famili: Poaceae Genus: TriticumL. Spesies T. aestivum T. aethiopicum T. araraticum T. boeoticum T. carthlicum T.compactum T. dicoccoides T. dicoccon T. durum T. ispahanicum T. karamyschevii T. macha T. militinae T. monococcum T. polonicum T. spelta T. sphaerococcum T. timopheevii T. turanicum T. turgidum T. urartu T. vavilovii T. zhukovsky...
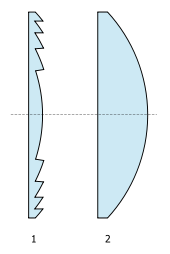
Lantern using a Fresnel lens A Fresnel with the lens open to show the ridges. There is no lamp in the instrument A Fresnel lantern (pronounced frəˈnɛl or fruh-nel) is a common lantern used in theatre that employs a Fresnel lens to wash light over an area of the stage. The lens produces a wider, soft-edged beam than a spotlight or key light, and is commonly used for back light and top light. Description 1: Cross section of a Fresnel lens 2: Cross section of a conventional plano-convex lens ...

Stasiun Serang LM06 Tampak depan bangunan Stasiun Serang.LokasiJalan Ki Tapa No. 2Cimuncang, Serang, Serang, Banten 42111IndonesiaKoordinat6°6′59″S 106°9′20″E / 6.11639°S 106.15556°E / -6.11639; 106.15556Koordinat: 6°6′59″S 106°9′20″E / 6.11639°S 106.15556°E / -6.11639; 106.15556Ketinggian+17 mOperator KAI Commuter Letakkm 113+446 lintas Angke–Tanah Abang–Rangkasbitung–Merak[1] Jumlah peron2 (satu peron sisi...

Pasal 301 adalah sebuah pasal di dalam Kitab Undang-Undang Hukum Pidana Turki yang melarang penghinaan terhadap Turki, bangsa Turki, lembaga pemerintahan Turki, atau pahlawan nasional Turki seperti Mustafa Kemal Atatürk. Pasal ini mulai berlaku pada 1 Juni 2005, dan diperkenalkan sebagai bagian dari paket reformasi hukum pidana dalam proses sebelum pembukaan perundingan keanggotaan Turki di Uni Eropa (UE), untuk membawa Turki memenuhi standar UE.[1][2] Versi asli pasal terseb...

American luxury department store Bergdorf redirects here. For other uses, see Bergdorf (disambiguation). Bergdorf Goodman Inc.The first Bergdorf Goodman storeCompany typeSubsidiaryIndustryRetailGenreDepartment storesFounded1899; 125 years ago (1899) in New York City, U.S.FounderHerman BergdorfHeadquarters754 5th Ave, New York, NY USA 1001940°45′48″N 73°58′27″W / 40.7634°N 73.9741°W / 40.7634; -73.9741Number of locations2 (both on 700 block...

Basketball player and coach (born 1962) Kim MulkeyMulkey in 2024Current positionTitleHead coachTeamLSUConferenceSECRecord91–14 (.867)Biographical detailsBorn (1962-05-17) May 17, 1962 (age 61)Santa Ana, California, U.S.Playing career1980–1984Louisiana Tech1983–1984USA National Team Position(s)Point guardCoaching career (HC unless noted)1985–1996Louisiana Tech (assistant)1996–2000Louisiana Tech (associate HC)2000–2021Baylor2021–presentLSU Head coaching recordOverall723–118...

For similarly titled films, see Jinn (disambiguation). 2013 Emirati filmDjinnTheatrical release posterDirected byTobe HooperWritten byDavid TullyProduced byDaniela TullyTim SmytheStarringKhalid LaithRazane JammalCinematographyJoel RansomEdited byAndrew CohenMark StevensMusic byBC SmithProductioncompaniesImage NationFilmWorksRelease date 25 October 2013 (2013-10-25) (Abu Dhabi Film Festival) Running time85 minutesCountryUnited Arab EmiratesLanguagesArabicEnglishBudgetUS$5 mi...

Regiment Louw Wepener / Oos VrystaatRegiment Louw Wepener / Oos Vrystaat EmblemActive1934Allegiance Allied forces South Africa Branch South African Army SizeBattalionPart of South African Infantry Corps Army Conventional Reserve Garrison/HQLadybrand BethlehemMotto(s)Ons vir jou Suid Afrika (Us for you, South Africa)Battle honours Military unit Regiment Louw Wepener was an infantry battalion of the South African Army. As a reserve force unit, it had a status roughly equi...

Formule E Généralités Sport Compétition automobile Création Lancement du projet : 2011Première saison : 2014Statut mondial : 2021 Organisateur(s) FIAFormula E Holdings Éditions 9e Catégorie Monoplace électrique Périodicité Annuelle Nations 9 Participants 25 pilotes11 équipes Épreuves 11 Nombre de manches 16 Site web officiel www.fiaformulae.com Palmarès Champion pilote Jake Dennis Champion constructeur Envision Racing Plus titré(s) Pilotes : Jean-Éric Verg...

Racconti d'estateLingua originaleitaliano Paese di produzioneItalia, Francia Anno1958 Durata113 min Dati tecniciEastmancolorrapporto: 2,35:1 Generecommedia RegiaGianni Franciolini SoggettoEdoardo Anton, Alberto Moravia, Alberto Sordi, Gianni Franciolini, René Barjavel, Rodolfo Sonego, Ennio Flaiano, Sergio Amidei SceneggiaturaEdoardo Anton, Alberto Moravia, Alberto Sordi, Gianni Franciolini, René Barjavel, Rodolfo Sonego, Ennio Flaiano, Sergio Amidei ProduttoreMario Cecchi Gori Casa di ...

† Палеопропитеки Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:СинапсидыКласс:�...

此条目页的主題是香港九龍的渡船街。关于其他地方的同名街道,請見「渡船街」。 Ferry Street渡船街渡船街與西九龍走廊的交匯路段,此段連同渡船街天橋隸屬於5號幹線。命名緣由命名文件:1941年10月24日憲報第1260號政府公告、1947年5月23日憲報第431號政府公告、1975年3月14日憲報第585號政府公告、2020年10月16日憲報第5984號政府公告命名日期1941年10月24日[1]道路...

「アプリケーション」はこの項目へ転送されています。英語の意味については「wikt:応用」、「wikt:application」をご覧ください。 この記事には複数の問題があります。改善やノートページでの議論にご協力ください。 出典がまったく示されていないか不十分です。内容に関する文献や情報源が必要です。(2018年4月) 古い情報を更新する必要があります。(2021年3月)出...

本表是動態列表,或許永遠不會完結。歡迎您參考可靠來源來查漏補缺。 潛伏於中華民國國軍中的中共間諜列表收錄根據公開資料來源,曾潛伏於中華民國國軍、被中國共產黨聲稱或承認,或者遭中華民國政府調查審判,為中華人民共和國和中國人民解放軍進行間諜行為的人物。以下列表以現今可查知時間為準,正確的間諜活動或洩漏機密時間可能早於或晚於以下所歸�...

AngakauitaiPulau AngakauitaiAngakauitaiGeografiLokasiSamudera PasifikKoordinat23°9′55″S 135°2′6″W / 23.16528°S 135.03500°W / -23.16528; -135.03500Koordinat: 23°9′55″S 135°2′6″W / 23.16528°S 135.03500°W / -23.16528; -135.03500KepulauanTuamotuLuas0.7 km2Titik tertinggi(tanpa nama) (139 m)PemerintahanNegara PrancisKolektivitas Polinesia PrancisAdministrative subdivisionTuamotuKomuneKepulauan Gam...

إقليم بوجدور إقليم بوجدور تقسيم إداري البلد المغرب [1] العاصمة بوجدور التقسيم الأعلى جهة العيون الساقية الحمراء خصائص جغرافية إحداثيات 26°08′00″N 14°30′00″W / 26.133333333333°N 14.5°W / 26.133333333333; -14.5 المساحة 43753 كيلومتر مربع السكان التعداد السكاني 50556...

The first railway signalling in Greece was installed on the Athens–Piraeus Railway at the turn of the 20th century, when semaphores and boards were added with the line's electrification. Other Greek trains at that time were controlled by signals given manually by station masters. During World War II, German occupation forces installed mechanically operated semaphore signals at the entrance to all stations, with some light signals at busy stations. Modern signalling is provided through colou...

Hair and cosmetic treatment salon This article is about the establishment where hair is cut and styled. For the type of gathering, see Salon (gathering). Not to be confused with beauty store. This article may require cleanup to meet Wikipedia's quality standards. The specific problem is: Outdated content and references, lacking information and (reliable) inline citations, Verify citations; Remove uncited opinion/narratives; needs re-organization; What version of English? Consider TNT? Please ...

Mountain in the state of Colorado, United States Crestone PeakCrestone Peak seen from Kit CarsonHighest pointElevation14,300 feet (4,359 m)[1]NAVD88Prominence4,554 ft (1,388 m)[1]Isolation27.4 mi (44.1 km)[1]ListingNorth America highest peaks 36thU.S. highest major peaks 22ndColorado highest major peaks 7thColorado fourteeners 7thColorado county high points 7thCoordinates37°58′00″N 105°35′07″W / 37.9666665°N 105....
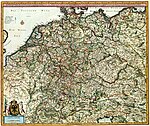
This article is about the debate over responsibility for World War I. For the notion of collective guilt for the Holocaust, see German collective guilt. European diplomatic alignments shortly before the war. The Ottomans joined with Germany shortly after the war started. Italy remained neutral in 1914 and joined the Entente in 1915. Part of a series on the History of Germany Topics Chronology Historiography Military history Economic history Healthcare Islam LGBT history Jewish history Women's...
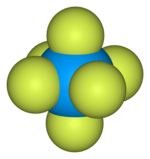
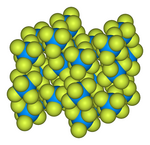





![Ball-and-stick model of the unit cell of uranium hexafluoride[8]](http://upload.wikimedia.org/wikipedia/commons/thumb/c/c6/Uranium-hexafluoride-unit-cell-3D-balls.png/180px-Uranium-hexafluoride-unit-cell-3D-balls.png)
![Bond lengths and angles of gaseous uranium hexafluoride[9]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Uranium_hexafluoride_dimensions.svg/180px-Uranium_hexafluoride_dimensions.svg.png)