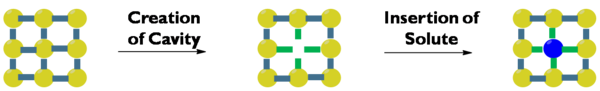
Solvation
|
Read other articles:

Questa voce o sezione sull'argomento centri abitati della California non cita le fonti necessarie o quelle presenti sono insufficienti. Puoi migliorare questa voce aggiungendo citazioni da fonti attendibili secondo le linee guida sull'uso delle fonti. Segui i suggerimenti del progetto di riferimento. Downeycomune Downey – VedutaIl municipio. LocalizzazioneStato Stati Uniti Stato federato California ConteaLos Angeles AmministrazioneSindacoRick Trejo TerritorioCoordinate33°56�...

Halaman judul Constitutio Criminalis Theresiana Constitutio Criminalis Theresiana (juga disebut Nemesis Theresiana atau Theresiana saja) adalah kitab hukum pidana yang dikeluar oleh penguasa Monarki Habsburg Maria Theresia. Kitab ini berisi hukum pidana acara maupun substantif dan berlaku di seluruh wilayah Austria dan Bohemia. Namun, kitab pidana ini tidak berlaku di Hungaria. Kitab ini dirancang di Wina pada tahun 1768, dan pada tanggal 31 Desember pada tahun yang sama mulai berlaku (walaup...

Istana Lateran dengan Obelisk Thutmosis III dan (kanan) paviliun Basilika Santo Yohanes Lateran, lukisan pensil dari abad ke-18 Istana Lateran adalah sebuah istana kuno dari zaman Kekaisaran Romawi dan kemudian menjadi salah satu Istana Kepausan. Istana ini berdekatan dengan Basilika Santo Yohanes Lateran, sebuah gereja katedral di Roma, Italia. Pada saat ini, Istana Lateran menjadi Musium Kepausan untuk Kristen Kuno. Sejak abad ke-4, Istana Lateran di Piazza San Giovanni di tenggara Roma ini...

Bagian dari seriPendidikan di Indonesia Kementerian Pendidikan, Kebudayaan, Riset, dan Teknologi Republik Indonesia Pendidikan anak usia dini TK RA KB Pendidikan dasar (kelas 1–6) SD MI Paket A Pendidikan dasar (kelas 7–9) SMP MTs Paket B Pendidikan menengah (kelas 10–12) SMA MA SMK MAK SMA SMTK SMAK Utama Widya Pasraman Paket C Pendidikan tinggi Perguruan tinggi Akademi Akademi komunitas Institut Politeknik Sekolah tinggi Universitas Lain-lain Madrasah Pesantren Sekolah alam Sekolah ru...

County in Kansas, United States Not to be confused with Pawnee, Kansas. County in KansasPawnee CountyCountyPawnee County Courthouse in Larned (2009)Location within the U.S. state of KansasKansas's location within the U.S.Coordinates: 38°09′N 99°12′W / 38.15°N 99.2°W / 38.15; -99.2Country United StatesState KansasFoundedFebruary 26, 1867Named forPawnee tribeSeatLarnedLargest cityLarnedArea • Total755 sq mi (1,960 km2) �...

Appennino umbro-marchigianoI Sibillini visti dal versante marchigianoContinenteEuropa Stati Italia Catena principaleAppennino centrale (negli Appennini) Cima più elevataMonte Vettore (2.476 m s.l.m.) L'Appennino umbro-marchigiano è la sezione dell'Appennino centrale, compresa tra l'Appennino tosco-emiliano a nord e l'Appennino abruzzese a sud, che interessa l'Umbria e le Marche. La vetta più alta è il Monte Vettore (2.476 m s.l.m.). Indice 1 Descrizione 1.1 Confini 1.2...

Voce principale: Coppa delle Coppe 1988-1989. Finale della Coppa delle Coppe 1988-1989Il Wankdorfstadion di Berna, teatro della finaleInformazioni generaliSport Calcio CompetizioneCoppa delle Coppe 1988-1989 Data10 maggio 1989 ImpiantoStadio Wankdorf Spettatori45 000 Dettagli dell'incontro Barcellona Sampdoria 2 0 Arbitro George Courtney Successione ← Finale della Coppa delle Coppe 1987-1988 Finale della Coppa delle Coppe 1989-1990 → Modifica dati su Wi...

Ini adalah nama Batak Angkola, marganya adalah Pasaribu. H.Syahrul Martua PasaribuS.H. Bupati Tapanuli Selatan ke-20Masa jabatan17 Februari 2016 – 17 Februari 2021PresidenJoko WidodoGubernurTengku Erry NuradiR. Sabrina (Plh.)Eko Subowo (Pj.)Edy RahmayadiWakilAswin Efendi SiregarPendahuluSarmadan Hasibuan (Pj.)PenggantiDolly Putra Parlindungan PasaribuMasa jabatan12 Agustus 2010 – 12 Agustus 2015PresidenSusilo Bambang YudhoyonoJoko WidodoGubernurGatot Pujo NugrohoTeng...

The main fire station in Brighton was built in 1938 at Preston Circus. Brighton and Hove, a city and unitary authority in the English county of East Sussex, has a wide range of public services funded by national government, East Sussex County Council, Brighton and Hove City Council and other public-sector bodies. Revenue to fund these services comes partly from Council Tax, which is paid annually by residents: this tax provides the city council with nearly 20% of its income and also helps to...

33°20′00″N 44°23′00″E / 33.333333°N 44.383333°E / 33.333333; 44.383333 المملكة العراقية الهاشمية 1932 – 1958 المملكة العراقيةعلم المملكة العراقيةشعار خريطة المملكة العراقية عاصمة بغداد نظام الحكم ملكية دستورية اللغة الرسمية العربية اللغة العربية الديانة الإسلام الملك فيصل ال�...

Карта океанических бассейнов по результатам спутниковой альтиметрии. Видны структуры морского дна обнаруженные по гравитационному искажению уровня поверхности моря. (1995, NOAA) Высшая геодезия (теоретическая геодезия) — одно из основных направлений современной геодез...

Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. Le contenu de cet article ou de cette section est peut-être sujet à caution et doit absolument être sourcé (décembre 2014). Si vous connaissez le sujet dont traite l'article, merci de le reprendre à partir de sources pertinentes en utilisant notamment les notes de fin de page. Vous pouvez également laisser un mot d'explication en page de discussion. Woonerf aux Pays-Bas. Le terme woonerf provient de la lan...

Seven DaysTheatrical posterNama lainHangul세븐 데이즈 Alih Aksara yang DisempurnakanSebeun DeijeuMcCune–ReischauerSebŭn Teijŭ SutradaraWon Shin-yunProduserLee Seo-yeol Jeong Yong-wook Jeong Geun-hyeon Im Choong-geunDitulis olehYoon Jae-guPemeranYunjin KimPark Hee-soonKim Mi-sookPenata musikKim Jun-seongSinematograferChoi Young-hwanPenyuntingShin Min-kyungPerusahaanproduksiPrime EntertainmentYoon & Joon FilmsDistributorPrime EntertainmentTanggal rilis 14 November 2007&...
2020年夏季奥林匹克运动会波兰代表團波兰国旗IOC編碼POLNOC波蘭奧林匹克委員會網站olimpijski.pl(英文)(波兰文)2020年夏季奥林匹克运动会(東京)2021年7月23日至8月8日(受2019冠状病毒病疫情影响推迟,但仍保留原定名称)運動員206參賽項目24个大项旗手开幕式:帕维尔·科热尼奥夫斯基(游泳)和马娅·沃什乔夫斯卡(自行车)[1]闭幕式:卡罗利娜·纳亚(皮划艇)&#...

Winmarleigh is a civil parish in the Wyre district of Lancashire, England. It contains two listed buildings that are recorded in the National Heritage List for England. Both the listed buildings are designated at Grade II, the lowest of the three grades, which is applied to buildings of national importance and special interest.[1] Other than the village of Winmarleigh, the parish is rural. The listed buildings are a public house and a church. Map all coordinates using OpenStreet...

For other uses, see Chambers Street (disambiguation), Cortlandt Street (disambiguation), Park Place (disambiguation), and World Trade Center (disambiguation). New York City Subway station in Manhattan New York City Subway station in Manhattan, New York Chambers Street World Trade Center Park Place Cortlandt Street New York City Subway station complexPassageway between Eighth Avenue and Seventh Avenue linesStatio...

Political divisions of Ukraine Part of a series on theSubdivisions of Ukraine First level 1 autonomous republic 24 oblasts 2 cities with special status Second level 136 raions Third level 1469 hromadas This sidebar: viewtalkedit The administrative divisions of Ukraine (Ukrainian: Адміністративний устрій України, romanized: Administratyvnyi ustrii Ukrainy) are under the jurisdiction of the Ukrainian Constitution. Ukraine is a unitary state with three levels of...

مملكة تايلاند ราชอาณาจักรไทย (تايلندية) تايلاندعلم تايلاند تايلاندشعار تايلاند النشيد: نشيد تايلاند الوطني الأرض والسكان إحداثيات 14°N 101°E / 14°N 101°E / 14; 101 [1] أعلى قمة جبل دوى إن تانون أخفض نقطة خليج تايلاند (0 متر) المساحة 513...

Queen of Denmark and Norway from 1766 to 1772 Caroline Matilda of Great BritainPortrait by Jens Juel, 1771Queen consort of Denmark and NorwayTenure8 November 1766 – April 1772Coronation1 May 1767Born(1751-07-22)22 July 1751 (New Style)[a]Leicester House, London, England, Great BritainDied10 May 1775(1775-05-10) (aged 23)Celle, Holy Roman EmpireBurial13 May 1775Stadtkirche St. Marien, CelleSpouse Christian VII of Denmark (m. 1766; div. 17...

Disambiguazione – Se stai cercando il giorno del calendario gregoriano, vedi 18 dicembre. Disambiguazione – Se stai cercando la piazza di Torino dedicata alla strage di Torino del 1922, su cui si trova la stazione di questa voce, vedi Piazza XVIII Dicembre. XVIII Dicembre Stazione dellametropolitana di Torino GestoreGTT Inaugurazione2006 StatoIn uso LineaM1 LocalizzazionePiazza XVIII Dicembre, Torino TipologiaStazione sotterranea InterscambioTram e bus urbani ed extraurbani Dintorni(in o...