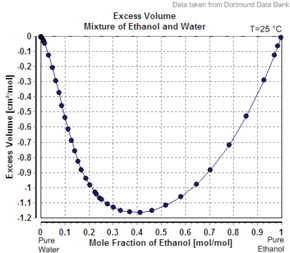
Apparent molar property
|
Read other articles:

Gunnar SjöbergLahir(1909-03-25)25 Maret 1909Stockholm, SwediaMeninggal8 Juni 1977(1977-06-08) (umur 68)SwediaTahun aktif1937-1966 Gunnar Sjöberg (25 Maret 1909 – 8 Juni 1977) adalah seorang pemeran film Swedia.[1] Ia lahir di Stockholm, Swedia. Referensi ^ Gunnar Sjöberg. Swedish Film Database. Diarsipkan dari versi asli tanggal 2016-04-08. Diakses tanggal 6 February 2013. Pranala luar Gunnar Sjöberg di IMDb (dalam bahasa Inggris) Gunnar Sjöberg p...

Direktorat Jenderal Bimbingan Masyarakat Islam Kementerian Agama Republik IndonesiaSusunan organisasiDirektur JenderalProf. Dr. Phil. H. Kamaruddin Amin, MA.[1][2]Sekretaris DitjenH. Muhammad Fuad, S.Sos, M.Sc.Direktur Urusan Agama Islam dan Pembinaan SyariahDr. H. Adib, M.Ag.Direktur Bina Kantor Urusan Agama dan Keluarga SakinahH. Zainal Mustamin, S.Ag, MA.Direktur Penerangan Agama IslamDr. H. Ahmad Zayadi, M.Pd.Direktur Pemberdayaan Zakat dan WakafDrs. H. Tarmizi, MA. Kantor...

Dalam nama Korean ini, nama keluarganya adalah Jung. Jung Sung-hwaJung pada tahun 2013Lahir2 Januari 1975 (umur 49)Incheon, Korea SelatanPekerjaanPemeranTahun aktif1993–sekarang Nama KoreaHangul정성화 Alih AksaraJeong Seong-hwaMcCune–ReischauerChŏng Sŏng-hwa Jung Sung-hwa (Hangul: 정성화; lahir 2 Januari 1975) adalah pemeran Korea Selatan. Ia paling dikenal di teater musikal, dan membintangi produksi Korea dari Man of La Mancha dan Les Misérables.[1] ...
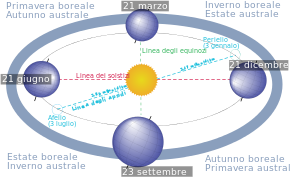
Disambiguazione – Se stai cercando altri significati, vedi Autunno (disambigua). Un paesaggio autunnale. L'autunno è una delle quattro stagioni in cui è suddiviso l'anno, segue l'estate e precede l'inverno.[1] Indice 1 Durata 2 La stagione 3 Nella cultura di massa 4 Etimologia 5 Note 6 Voci correlate 7 Altri progetti 8 Collegamenti esterni Durata Le posizioni del Sole e della Terra nell'arco delle stagioni. Il tramonto nel giorno dell'equinozio autunnale. Tipico autunno in Brasil...

Japanese samurai (1828–1877) who led the Satsuma Rebellion In this Japanese name, the surname is Saigō. This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (January 2018) (Learn how and when to remove this template message) Saigō TakamoriA portrait of Takamori by Ishikawa ShizumasaNative name西郷 隆盛Birth nameSaigō KokichiOther name(s)Saigō NanshūSaig...

Padma VibhushanJenisSipilKategoriNasionalDiinstitusikan1954Penghargaan pertama1954Penghargaan terakhir2016Total yang diberi penghargaan294Dianugerahi olehPemerintah IndiaNama sebelumnyaPadma Vibhushan Pahela Warg (Kelas I)ObverseSebuah bunga teratai yang berada di bagain tengah dan teks Padma yang ditulis dalam aksara Devanagari ditempatkan di bagian atas dan teks Vibhushan ditempatkan di bagian bawah teratai tersebut.ReverseSebuah Lambang India platinum ditempatkan di bagian tengah...

Gorge in northern Thessaly, Greece Vale of TempeΚοιλάδα των ΤεμπώνView of the Pineios River gorge.Vale of TempeΚοιλάδα των ΤεμπώνThessaly, GreeceFloor elevationapprox. 267 metres (876 ft)Length12.4 kilometres (7.7 mi)Width0.7 to 1.5 kilometres (0.43 to 0.93 mi)GeographyCoordinates39°52′37″N 22°33′58″E / 39.877°N 22.566°E / 39.877; 22.566 RiversPineios River The Vale of Tempe (/ˈtɛmpi/;[1] Greek:...

Association football club in England Football clubLichfield CityFull nameLichfield City Football ClubFounded1970[1]GroundCity Ground, LichfieldCapacity1,500[1]ChairmanDarren LeaverManagerIvor GreenLeagueMidland League Premier Division2022–23Midland League Premier Division, 11th of 20 Home colours Lichfield City Football Club is a football club based in Lichfield, Staffordshire, England. They are members of the Midland League Premier Division and play at the City Ground. Hist...

Area of an airplane where workers can rest in private A multiple-bunk Class 1 crew rest compartment. A crew rest compartment is a section of an airliner dedicated for breaks and sleeping by crew members during off-duty periods.[1][2] Federal Aviation Regulations have provisions requiring crew rest areas be provided in order to operate a long-haul flight by using multiple crew shifts.[3] Passengers are restricted from accessing crew rest compartments by regulations; the...

River in north India For other uses, see Beas (disambiguation). Beas RiverVyas RiverThe Beas River in Himachal PradeshThe Beas river flows into the Satluj and feeds into the Indus ([1])LocationCountryIndiaStateHimachal Pradesh, PunjabPhysical characteristicsSourceBeas Kund • locationHimalayas, Himachal Pradesh • coordinates32°21′59″N 77°05′08″E / 32.36639°N 77.08556°E / 32.36639; 77.08556 MouthSutlej River •&#...

V. Ramaswamy Aiyer V. Ramaswamy Aiyer (4 August 1871 – 22 January 1936) was a civil servant in the Madras Provincial Service. In 1907, along with a group of friends, he founded the Indian Mathematical Society with headquarters in Pune. He was the first Secretary of the Society and acted in that position until 1910. Ramaswamy Aiyer also served the Society as its President from 1926 to 1930.[1] Early life and career Ramaswamy Aiyer was born on 4 August 1871 at Satyamangalam, in t...

Piscina Guido Romano Informazioni generaliStato Italia UbicazioneVia Ampère, 20 Milano Inizio lavori1929 Inaugurazione1929 ProprietarioComune di Milano ProgettoLuigi Lorenzo Secchi Informazioni tecnicheStrutturapianta rettangolare Dim. vasca100 × 40 m Uso e beneficiariSport acquaticiMilanoSport Mappa di localizzazione Modifica dati su Wikidata · Manuale La Piscina Romano è una struttura sportiva scoperta sita a Milano, in via Ampère, inaugurata il 28 luglio 1929. È intito...

لمعانٍ أخرى، طالع أصلة (توضيح). أصلة فوق بوابة قلعة بيلفيدير، في سنترال بارك في مدينة نيويورك. الأصَلَة[1] وتعرف أيضًا باسم أمّ طَبَق[2][1] أو الصل أو الملكة أو المكللة هي حية ورد ذكرها في أساطير الأولين لا يعلم ما هي، أما الأصلة في أيامنا فهي حية.[3] وقال ا...

العلاقات الغينية الماليزية غينيا ماليزيا غينيا ماليزيا تعديل مصدري - تعديل العلاقات الغينية الماليزية هي العلاقات الثنائية التي تجمع بين غينيا وماليزيا.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين: وجه المقارنة غين�...

American college basketball season 2016–17 Drake Bulldogs men's basketballConferenceMissouri Valley ConferenceRecord7–24 (5–13 MVC)Head coachRay Giacoletti (8 games)Jeff Rutter (interim)Assistant coaches Dave Buchanan JR Blount Home arenaKnapp CenterSeasons← 2015–162017–18 → 2016–17 Missouri Valley Conference men's basketball standings vte Conf Overall Team W L PCT W L PCT Illinois State 17 – 1 .944 28 –...

Chanoch NissanyNissany memakai sebuah topi Minardi.Kebangsaan Israel Hungaria(kewarganegaraan ganda)Lahir29 Juli 1963 (umur 60)Tel Aviv, IsraelTerkait denganRoy Nissany (anak)Ajang sebelumnyaInternational Formula 3000 (2004)World Series Lights (2003)FIA CEZHungarian championships (2002–11, 2013–14)Gelar juara2002–03, 2003–04, 2006–07, 2009FIA CEZHungarian championships Chanoch Nissany (bahasa Ibrani: חנוך ניסני, lahir 29 Juli 1963) merupakan seorang pengusah...

2020 song by Taylor Swift MarjorieSong by Taylor Swiftfrom the album Evermore ReleasedDecember 11, 2020Recorded2020Length4:17LabelRepublicSongwriter(s) Taylor Swift Aaron Dessner Producer(s)Aaron DessnerLyric videoMarjorie on YouTube Marjorie is a song by the American singer-songwriter Taylor Swift from her ninth studio album, Evermore (2020). A tribute to Swift's late maternal grandmother, the opera singer Marjorie Finlay, the song features bits of advice that Finlay offered to Swift and tou...

Part of Lionsgate films that specializes in direct to video Lionsgate PremiereCompany typeDivisionIndustryMotion pictureFounded2015; 9 years ago (2015)HeadquartersSanta Monica, California, U.S.Key people Jean McDowell Tim Pallen Adam Sorensen ProductsFilm distributionParentLionsgate FilmsWebsitelionsgate.com Lionsgate Premiere is the speciality film division of entertainment company Lionsgate Films that specializes in direct-to-video and direct-to-video on demand.[1]...

Renault 4CV Datos generalesOtros nombres Hino 4CVRenault 4/4Renault 760Renault 750Fabricante RenaultFábricas Billancourt, FranciaValladolid (FASA), EspañaHino, JapónSídney, Australia, Canadá[1]Período 1947-1961ConfiguraciónTipo Automóvil de turismoCarrocerías Sedán cuatro puertasConfiguración Motor trasero / tracción traseraDimensionesLongitud 3663 mmAnchura 1430 mmAltura 1450 mmDistancia entre ejes 2096 mmPeso 560-620 kgOtros modelosSimilares Seat 600Citroën 2CVPeugeot 20...

الاندماج والاستحواذ (بالإنجليزية: Mergers and acquisitions) (ويختصر M&A) يشير إلى جانب من إستراتيجية الشركات، تمويل الشركات وإدارة التعامل من بيع، شراء، تقسيم والجمع بين مختلف الشركات والكيانات المماثلة والتي يمكن أن تساعد أي مؤسسة لتنمو بسرعة في قطاعها أو في مكان المنشأ، أو في حقل...