Stibine
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

President of theUniversity of WashingtonIncumbentAna Mari Caucesince October 13, 2015 (2015-10-13)University of WashingtonResidenceHill-CrestAppointerBoard of Regents of the University of WashingtonFormationNovember 4, 1861 (1861-11-04)First holderAsa MercerSalary$714,300[1]WebsiteOffice of the President The President of the University of Washington is the chief administrator of the university. The University of Washington is a public university in ...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Oktober 2022. Artikel ini membutuhkan rujukan tambahan agar kualitasnya dapat dipastikan. Mohon bantu kami mengembangkan artikel ini dengan cara menambahkan rujukan ke sumber tepercaya. Pernyataan tak bersumber bisa saja dipertentangkan dan dihapus.Cari sumber: ...

An EducationPoster film An EducationSutradaraLone ScherfigProduserFinola DwyerAmanda PoseyDitulis olehNick HornbyBerdasarkanAn Educationoleh Lynn BarberPemeranCarey MulliganPeter SarsgaardAlfred MolinaRosamund PikeDominic CooperEmma ThompsonOlivia WilliamsPenata musikPaul EnglishbySinematograferJohn de BormanPenyuntingBarney PillingPerusahaanproduksiBBC FilmsFinola Dwyer ProductionsWildgaze FilmsEndgame EntertainmentDistributorSony Pictures ClassicsTanggal rilis 18 Januari 2009 (20...

Italian-American physicist Alessandra LanzaraNationalityItalian, USAlma materUniversity of Rome La SapienzaScientific careerFieldsCondensed Matter PhysicsInstitutionsUniversity of California, Berkeley Lawrence Berkeley National Laboratory Alessandra Lanzara is an Italian-American physicist and the distinguished Charles Kittel Professor of physics at the University of California, Berkeley[1] since 2002, where she leads an experimental materials physics group. She is the founding d...

55°02′06″N 7°17′02″W / 55.035°N 7.284°W / 55.035; -7.284 Grammar school in Derry, Northern IrelandThornhill CollegeColáiste Chnoc na nDealg[1]AddressCulmore RoadDerry, BT48 8JFNorthern IrelandInformationTypeGrammar schoolMottoAdveniat Regnum Tuum (Thy Kingdom come)Religious affiliation(s)Roman CatholicEstablished1886Local authorityEducation Authority (Western)PrincipalSharon Mallett, BSSc PGDFHE MSc PQMStaff100Grades6-12GenderGirlsAge11 to 18En...

This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (January 2009) (Learn how and when to remove this message) 1968 studio album by The Electric PrunesRelease of an OathStudio album by The Electric PrunesReleasedNovember 1968 (1968-11)Recorded1968GenrePsychedelic rockLength24:56LabelRepriseProducerDavid HassingerThe Electric Prunes chronology Mas...

土库曼斯坦总统土库曼斯坦国徽土库曼斯坦总统旗現任谢尔达尔·别尔德穆哈梅多夫自2022年3月19日官邸阿什哈巴德总统府(Oguzkhan Presidential Palace)機關所在地阿什哈巴德任命者直接选举任期7年,可连选连任首任萨帕尔穆拉特·尼亚佐夫设立1991年10月27日 土库曼斯坦土库曼斯坦政府与政治 国家政府 土库曼斯坦宪法 国旗 国徽 国歌 立法機關(英语:National Council of Turkmenistan) ...

British politician and adventurer (born 1974) This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This biography of a living person needs additional citations for verification. Please help by adding reliable sources. Contentious material about living persons that is unsourced or poorly sourced must be removed immediately from the article and its talk page, especially if potentially libelous.F...

烏克蘭總理Прем'єр-міністр України烏克蘭國徽現任杰尼斯·什米加尔自2020年3月4日任命者烏克蘭總統任期總統任命首任維托爾德·福金设立1991年11月后继职位無网站www.kmu.gov.ua/control/en/(英文) 乌克兰 乌克兰政府与政治系列条目 宪法 政府 总统 弗拉基米尔·泽连斯基 總統辦公室 国家安全与国防事务委员会 总统代表(英语:Representatives of the President of Ukraine) 总...

المستشار أنغيلا ميركل استضافة القمة G8 في هيليجندام ألمانيا دولة أوروبية تقع في وسط اوروربا وهي عضو في مجموع الأربعة والثمانية والعشرين ومنظمة التنمية والتعاون الاقتصادي.[1] كما لديها شبكة من 229 بعثة دبلوماسية في الخارج. وترتبط بعلاقات مع أكثر من 190 دولة. اعتبارًا من عام ...

伊斯兰合作组织Organisation of Islamic Cooperation(英語)Organisation de la Coopération Islamique(法語)منظمة التعاون الإسلامي(阿拉伯語) 旗帜格言:To safeguard the interests and ensure the progress and well-being of Muslims 成员国 观察国 暂停会籍行政总部 沙地阿拉伯吉达 官方语言阿拉伯语英语法语类型宗教成员国57个在籍成员国(英语:Member states of the Organisation ...

Д. Э. Милле - Роялист вне закона, 1853 Проскри́пция (лат. proscriptio от proscribere — «письменно обнародовать, оглашать») — в Древнем Риме — список лиц, объявленных вне закона. За выдачу или убийство включённого в списки назначалась награда, за укрывательство — казнь. Иму...

King of Alasora, founder of the Imerina Kingdom (fl. 1540–1575) AndriamaneloKing of AlasoraReignc. 1540–1575PredecessorRafohy (or Rangita)SuccessorRalamboBornMerimanjakaDiedc. 1575AlasoraBurialAlasoraSpouseRamaitsoanalaIssue(6 stillborn/died in infancy)RalamboDynastyHova dynastyFatherManelobeMotherRafohy (or Rangita) Andriamanelo (fl. 1540–1575) was king of Alasora in the central highlands region of Madagascar. He is generally considered by historians to be the founder of the Ki...
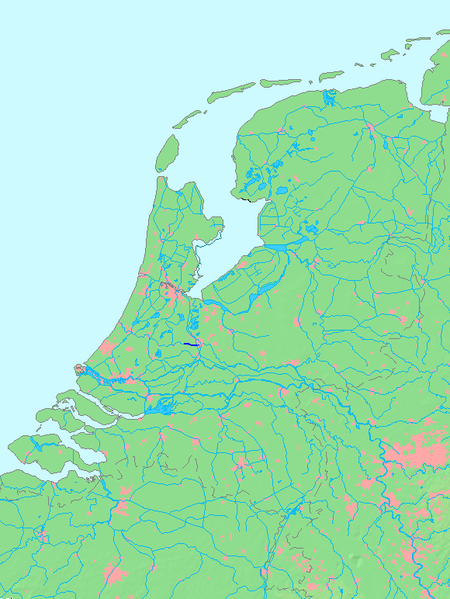
River in Utrecht, NetherlandsLeidse RijnLeiden's RhineLeidse Rijn between Harmelen and De MeernLocation of Leidse Rijn in dark blue.LocationCountryNetherlandsProvinceUtrechtPhysical characteristicsSourceKromme Rijn • locationUtrecht, Utrecht • coordinates52°05′33″N 5°06′38″E / 52.09250°N 5.11056°E / 52.09250; 5.11056 MouthOude Rijn • locationHarmelen, South Holland • coordinates52°05′31...

ملح الإحداثيات 32°30′22″N 36°51′07″E / 32.506111°N 36.851944°E / 32.506111; 36.851944 تقسيم إداري البلد سوريا التقسيم الأعلى محافظة السويداءناحية ملح تعديل مصدري - تعديل لمعانٍ أخرى، طالع ملح (توضيح). ملح بلدة وناحية سوريّة إداريّة تتبع منطقة صلخد في محافظة ال�...

La Gleva localidadPaís España• Com. autónoma Cataluña• Provincia Barcelona• Comarca Osona• Municipio Las Masías de VoltregáPoblación 1050 hab. (INE 2013)Código postal 08508[editar datos en Wikidata] La Gleva es una localidad que forma parte del municipio de Las Masías de Voltregá, en la comarca de Osona, provincia de Barcelona. Se halla junto al río Ter en el lugar en que éste hace de límite con el munic...

Nature reserve in Stoke-on-Trent, UK Bagnall Road WoodWoodland walkLocation in StaffordshireLocationNear Milton, StaffordshireOS gridSJ 912 501Coordinates53°2′54″N 2°7′58″W / 53.04833°N 2.13278°W / 53.04833; -2.13278Area6.11 hectares (15.1 acres)Operated byStoke-on-Trent City CouncilDesignationLocal nature reserveWebsiteBagnall Road Wood Bagnall Road Wood is a local nature reserve near Milton, on the eastern fringe of Stoke-on-Trent, England. History a...

Division of the Pali Canon of Theravada Buddhism Sutta PiṭakaTypeCanonical textParent CollectionTipitakaContainsDīgha Nikāya, Majjhima Nikāya, Saṃyutta Nikāya, Aṅguttara Nikāya, Khuddaka NikāyaPāli literature Pāli Canon 1. Vinaya Piṭaka 1. Suttavibhaṅga 2. Khandhaka 3. Parivāra 2. Sutta Piṭaka 1. Dīgha Nikāya 2. Majjhima Nikāya 3. Saṃyutta Nikāya 4. Aṅguttara Nikāya 5. Khuddaka Nikāya KhuddakapāṭhaDhammapadaUdānaItivuttakaSuttanipātaVimānavatthuPetavatthu...

Cet article est une ébauche concernant une chronologie ou une date et le pays de Galles. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Chronologie du pays de Galles 2007 2008 2009 2010 2011 2012 2013 2014 2015 Chronologies Données clés 2008 2009 2010 2011 2012 2013 2014Décennies :1980 1990 2000 2010 2020 2030 2040Siècles :XIXe XXe XXIe XXIIe XXIIIeMillénaires...

Aix-en-Pévèlecomune Aix-en-Pévèle – Veduta LocalizzazioneStato Francia RegioneAlta Francia Dipartimento Nord ArrondissementDouai CantoneOrchies TerritorioCoordinate50°29′N 3°17′E50°29′N, 3°17′E (Aix-en-Pévèle) Altitudine22 e 47 m s.l.m. Superficie6,53 km² Abitanti1 104[1] (2009) Densità169,07 ab./km² Altre informazioniCod. postale59310 Fuso orarioUTC+1 Codice INSEE59004 CartografiaAix-en-Pévèle Sito istituzionaleModifica dati su ...





