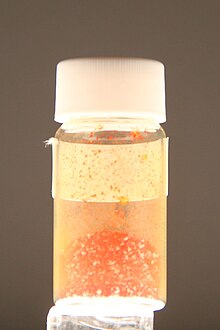
Antimony triiodide
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Novelist from Trinidad and Tobago (born 1986) Kevin Jared HoseinBorn1986 (age 37–38)Chaguanas, Trinidad and TobagoEducationUniversity of the West Indies, St. AugustineOccupationAuthorHonoursCommonwealth Short Story Prize (2018) Kevin Jared Hosein (born 1986) is a Caribbean novelist and short-story writer from Trinidad and Tobago.[1][2] He is known for winning the 2018 Commonwealth Short Story Prize with his story Passage.[1] He also won the regional (Caribbe...

American screenwriters Frank and Doris HursleyNationalityAmericanOccupationScreenwriting duoKnown for Have Gun, Will Travel Search for Tomorrow General Hospital Frank HursleyBirth NameFrank M. HursleyBorn(1902-11-21)November 21, 1902CanadaDiedFebruary 3, 1989(1989-02-03) (aged 86)Santa Barbara, California, United StatesFamilyBridget Dobson (daughter) Doris HursleyBorn(1898-09-29)September 29, 1898Milwaukee, Wisconsin United StatesDiedMay 5, 1984(1984-05-05) (aged 85)Santa Barba...

Koji Nakajima Informasi pribadiNama lengkap Koji NakajimaTanggal lahir 20 Agustus 1977 (umur 46)Tempat lahir Prefektur Osaka, JepangPosisi bermain GelandangKarier senior*Tahun Tim Tampil (Gol)1996-2002 Vegalta Sendai 2003-2008 JEF United Chiba 2009-2013 Sanfrecce Hiroshima * Penampilan dan gol di klub senior hanya dihitung dari liga domestik Koji Nakajima (lahir 20 Agustus 1977) adalah pemain sepak bola asal Jepang. Karier Koji Nakajima pernah bermain untuk Vegalta Sendai, JEF United Ch...

保良局馬錦明夫人章馥仙中學Po Leung Kuk Mrs.Ma-Cheung Fook Sien College翻漆後的校舍東北面(2022年3月)地址 香港新界離島區大嶼山東涌富東邨类型津貼中學宗教背景無隶属保良局创办日期1997年学区香港離島區東涌校長柯玉琼女士副校长鄭健華先生,劉俊偉先生助理校长梁煥儀女士职员人数56人年级中一至中六学生人数約700人,24個班別校訓愛、敬、勤、誠校歌保良局屬下校歌�...

Chemical compound Calcium glucoheptonateClinical dataAHFS/Drugs.comInternational Drug NamesATC codeA12AA10 (WHO) Identifiers IUPAC name calcium (2R,3R,4S,5R,6R)-2,3,4,5,6,7-hexahydroxyheptanoate CAS Number29039-00-7 YPubChem CID28327DrugBankDB00326 NChemSpider16741933 YUNIIL11651398JCompTox Dashboard (EPA)DTXSID30951637 ECHA InfoCard100.044.880 Chemical and physical dataFormulaC14H26CaO16Molar mass490.424 g·mol−13D model (JSmol)Interactive image SMILES [Ca+2]....

هنودمعلومات عامةنسبة التسمية الهند التعداد الكليالتعداد قرابة 1.21 مليار[1][2]تعداد الهند عام 2011ق. 1.32 مليار[3]تقديرات عام 2017ق. 30.8 مليون[4]مناطق الوجود المميزةبلد الأصل الهند البلد الهند الهند نيبال 4,000,000[5] الولايات المتحدة 3,982,398[6] الإمار...

土库曼斯坦总统土库曼斯坦国徽土库曼斯坦总统旗現任谢尔达尔·别尔德穆哈梅多夫自2022年3月19日官邸阿什哈巴德总统府(Oguzkhan Presidential Palace)機關所在地阿什哈巴德任命者直接选举任期7年,可连选连任首任萨帕尔穆拉特·尼亚佐夫设立1991年10月27日 土库曼斯坦土库曼斯坦政府与政治 国家政府 土库曼斯坦宪法 国旗 国徽 国歌 立法機關(英语:National Council of Turkmenistan) ...
2020年夏季奥林匹克运动会波兰代表團波兰国旗IOC編碼POLNOC波蘭奧林匹克委員會網站olimpijski.pl(英文)(波兰文)2020年夏季奥林匹克运动会(東京)2021年7月23日至8月8日(受2019冠状病毒病疫情影响推迟,但仍保留原定名称)運動員206參賽項目24个大项旗手开幕式:帕维尔·科热尼奥夫斯基(游泳)和马娅·沃什乔夫斯卡(自行车)[1]闭幕式:卡罗利娜·纳亚(皮划艇)&#...

Disambiguazione – Se stai cercando altri significati, vedi Margherita di Valois (disambigua). Disambiguazione – Regina Margot rimanda qui. Se stai cercando opere narrative ispirate a questa figura, vedi La regina Margot. Margherita di ValoisMargherita di Valois, schizzo attribuito a François Clouet (circa 1572).Regina consorte di FranciaStemma In carica2 agosto 1589 –17 dicembre 1599 PredecessoreLuisa di Lorena-Vaudémont SuccessoreMaria de' Medici Regina consorte di Nava...

Children of ancient Rome Childbirth in ancient Rome was dangerous for both the mother and the child. Mothers usually would rely on religious superstition to avoid death. Certain customs such as lying in bed after childbirth and using plants and herbs as relief were also practiced. Midwives assisted the mothers in birth. Once children were born they wouldn’t be given a name until 8 or 9 days after their birth. The number depended on if they were male or female. Once the days had passed, the ...

دانباد تقسيم إداري البلد الهند [1] خصائص جغرافية إحداثيات 23°47′34″N 86°26′06″E / 23.79278°N 86.435°E / 23.79278; 86.435 المساحة 2052 كيلومتر مربع الارتفاع 222 متر معلومات أخرى الرمز البريدي 826001 الرمز الهاتفي 326 الرمز الجغرافي 1272979 تعديل مصدري - تعديل دا�...

Bài viết này có chứa kí tự ngữ âm IPA. Nếu không thích hợp hỗ trợ dựng hình, bạn có thể sẽ nhìn thấy dấu chấm hỏi, hộp, hoặc ký hiệu khác thay vì kí tự Unicode. Giới hàn lâm đã có nhiều nỗ lực trong việc phục nguyên hệ thống âm vị học của tiếng Hán thượng cổ thông qua bằng chứng văn liệu. Mặc dù hệ chữ tượng hình Hán văn không trực tiếp ký âm từ ngữ, các thành tố...

Highest court in the U.S. state of New Jersey Supreme Court of New JerseySeal of the Supreme Court of New JerseyRichard J. Hughes Justice Complex, seat of the court40°12′49″N 74°45′51″W / 40.213604°N 74.764119°W / 40.213604; -74.764119Established1947 (1947) (in current form)JurisdictionState of New JerseyLocationRichard J. Hughes Justice Complex, Trenton, New Jersey, U.S.Coordinates40°12′49″N 74°45′51″W / 40.213604°N 74.7641...

Kris Allen, pemenang American Idol musim kedelapan. American Idol musim kedelapan merupakan kelanjutan tayangan reality show dalam bidang bernyanyi yang diselenggarakan oleh Fox untuk kedelapan kalinya pada 2009.[1] Dalam kompetisi tersebut terdapat 13 finalis dan 4 orang juri, yakni Simon Cowell, Paula Abdul, Randy Jackson, dan Kara DioGuardi.[2] Keempat juri tersebut berperan untuk memberikan masukan serta komentar atas penampilan para finalis.[2] Finalis dalam ameri...

Regional railroad in the Northeastern United States Providence and Worcester RailroadProvidence and Worcester GE B40-8W leads a passenger excursion for railfans at Plainfield, Connecticut in 2012OverviewParent companyGenesee & WyomingHeadquarters381 Southbridge Street, Worcester, MassachusettsReporting markPW, PWRZLocaleConnecticut, Massachusetts, and Rhode Island; New York City and Long Island via trackage rightsDates of operation1847–TechnicalTrack gauge4 ft 8+1⁄2&...

Turbojet engine YJ93 YJ93-GE-3 engine at National Museum of the United States Air Force Type Turbojet National origin United States Manufacturer General Electric Aircraft Engines Major applications North American XB-70 Valkyrie Developed into General Electric GE4 YB-58 at Edwards AFB with GE J93 engine pod The General Electric YJ93 turbojet engine was designed as the powerplant for both the North American XB-70 Valkyrie bomber and the North American XF-108 Rapier interceptor. The YJ93 was a s...

Vaishnava Hindu sect Part of a series onAyyavazhi Theology Ekam Vethan Thirumal Sivan Vaikundar The Trinity ScripturesAkilathirattu Ammanai Akilam one Akilam two Akilam three Akilam four Akilam five Akilam six Akilam seven Akilam eight Akilam nine Akilam ten Akilam eleven Akilam twelve Akilam thirteen Akilam fourteen Akilam fifteen Akilam sixteen Akilam seventeen Arul Nool Ukappadippu Uccippadippu Nadutheervai Ula Pothippu Saattu Neettolai Patthiram Panchadevar Urppatthi Sivakanta Athikarappa...

Lotus 43Descrizione generaleCostruttore Lotus Cars CategoriaFormula 1 SquadraTeam Lotus Progettata daColin ChapmanMaurice Philippe SostituisceLotus 33 Sostituita daLotus 49 Descrizione tecnicaMeccanicaTelaioMonoscocca in alluminio MotoreBRM H-16 Dimensioni e pesiLunghezza4112 mm Larghezza1853 mm Altezza774 mm Passo2436 mm Peso567 kg Risultati sportiviPiloti Jim Clark Graham Hill Palmares Corse Vittorie Pole Giri veloci 1 La Lotus 43 è una vettura da Formula 1 progettata da Colin C...

Ein Autoradio ist ein Hörfunkempfänger, der für den Einbau in ein Kraftfahrzeug entwickelt wurde. Zu diesem Zweck ist es üblicherweise auf eine Betriebsspannung von 12 V (bei Lkw auch 24 Volt) ausgelegt und besitzt ein normiertes oder an den Fahrzeugtyp angepasstes Gehäuse. Autoradios müssen besonders kompakt gebaut und gegenüber Erschütterungen und Temperaturschwankungen unempfindlich sein, was den Einsatz von Heim-Rundfunkempfangsgeräten in Fahrzeugen ausschließt. Viele Gerä...

ゴビ砂漠ゴビ砂漠の範囲(黄色部分)長軸全長1,500 km (930 mi)幅800 km (500 mi)面積1,295,000 km2 (500,000 sq mi)名称現地語名戈壁 (沙漠)Gēbì (shāmò)Говь (ᠭᠣᠪᠢ) (日本語)地理国中国 および モンゴル州スフバートル県 および ウムヌゴビ県自治区内モンゴル自治区座標北緯42度35分 東経103度26分 / 北緯42.59度 東経103.43度 / 42.59; 103.43�...