Copper hydride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Balap sepeda motor – berita · surat kabar · buku · cendekiawan · JSTOR Pembalap sepeda motor Balap sepeda motor adalah olahraga otomotif yang menggunakan sepeda motor. Balap sepeda motor, khususnya balap...

NGC 2070 di konstelasi Dorado. NGC 2070, juga dikenal sebagai Caldwell 103, adalah gugus terbuka yang sangat besar dan kemungkinan Gugus Super Bintang di Awan Magellan Besar, yang berada di dalam Nebula Tarantula, dan bertanggung jawab atas R136, dan sumber energi dan kecerahan terbesarnya, dengan ribuan bintang masifnya itu adalah wilayah awan yang kaya, yang mencakup R136a1. NGC 2070 terletak di konatelasi Dorado. NGC 2070 adalah gugus bintang muda raksasa berada di pusat nebula, hanya beru...

VincenzoPoster promosiHangul빈센조 GenreDrama kejahatan Komedi hitamPembuatStudio Dragon tvNDitulis olehPark Jae-bumSutradaraKim Hee-wonPemeran Song Joong-ki Jeon Yeo-been Ok Taec-yeon Kim Yeo-jin Kwak Dong-yeon Jo Han-cul Negara asalKorea SelatanBahasa asliKorea ItaliaJmlh. episode20 + 1 spesialProduksiProduser eksekutifLee Jang-soo Jang Sae-jung[a]Rumah produksiLogos FilmDistributortvN NetflixAnggaran₩20 miliar[1](~US$18 juta)Rilis asliJaringantvNRilis20 Februari ...

本條目存在以下問題,請協助改善本條目或在討論頁針對議題發表看法。 此條目需要补充更多来源。 (2018年3月17日)请协助補充多方面可靠来源以改善这篇条目,无法查证的内容可能會因為异议提出而被移除。致使用者:请搜索一下条目的标题(来源搜索:羅生門 (電影) — 网页、新闻、书籍、学术、图像),以检查网络上是否存在该主题的更多可靠来源(判定指引)。 �...

Logo tvOne sejak 14 Februari 2023 Halaman ini memuat daftar acara yang ditayangkan tvOne. Acara saat ini NewsOne Kabar tvOne Breaking News tvOne Kabar Pagi (Setiap hari pkl 04.30 WIB) Kabar Arena (Setiap hari pkl 06.00 WIB dan Senin-Jumat pkl 23.30 WIB) Kabar Siang (Senin-Jumat pkl 11.00 WIB dan Sabtu-Minggu pkl 11.30 WIB) Kabar Pemilu (Senin-Jumat pkl 14.30 WIB) (ditayangkan selama Pemilu 2024) Ragam Perkara (Senin-Jumat pkl 10.30 WIB di siang hari dan Setiap Hari pkl 15.30 WIB di sore hari)...

This article relies excessively on references to primary sources. Please improve this article by adding secondary or tertiary sources. Find sources: 10th term Sejm and 11th term Senate of Poland – news · newspapers · books · scholar · JSTOR (November 2023) (Learn how and when to remove this template message) Legislature of the Republic of Poland 10th term Sejm & 11th term Senate of the Republic of Poland ←9th term Sejm and 10th term Senate 11...
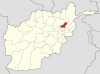
Mountain push in Afghanistan The Anjuman Pass (Persian: كتل انجمن Kotal-e Anjoman) (also written Anjoman Pass) (4,430 m) is a mountain pass in the Hindu Kush in Afghanistan. It connects the Panjshir Valley and beyond in the south-west with Badakhshan province and beyond to the north-east, which is the most north-easterly province of Afghanistan.[1][2][3] The Anjuman Pass is located on Panjshir Province's border with Badakhshan and Takhar province. The climate i...

Part of a series onSocialism HistoryOutline Development Age of the Enlightenment French Revolution Revolutions of 1848 Socialist calculation debate Socialist economics Ideas Calculation in kind Collective ownership Cooperative Common ownership Critique of political economy Economic democracy Economic planning Equal liberty Equal opportunity Free association Freed market Industrial democracy Input–output model Internationalism Labor-time calculation Labour voucher Material balance planning P...

Batman & Robin: Music from and Inspired by the Batman & Robin Motion PictureSoundtrack album by various artistsReleasedJune 10, 1997[1]RecordedSeptember 1996–March 1997Genre Alternative rock pop rock R&B hip hop electronica techno Length67:06LabelWarner Bros.Batman soundtracks chronology Batman Forever (score)(1995) Batman & Robin: Music from and Inspired by the Batman & Robin Motion Picture(1997) Batman Begins(2005) Singles from Batman & Robin The End Is...

American country musician This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (January 2024) (Learn how and when to remove this message) Skinny DennisBackground informationBirth nameDennis SanchezBorn(1946-09-03)September 3, 1946DiedMarch 20, 1975(1975-03-20) (aged 28)Sunset Beach, California, United StatesGenresCountryFolkInstrument(s)Double bassYears active...

American baseball player Baseball player Jake KalishKalish with the Omaha Storm Chasers in 2017Uni-President Lions – No. 27PitcherBorn: (1991-07-09) July 9, 1991 (age 32)Red Bank, New JerseyBats: SwitchThrows: Left Jacob Louis Kalish (born July 9, 1991) is an American professional baseball pitcher for the Uni-President Lions for the Chinese Professional Baseball League (CPBL). He was picked by the Royals in the 32nd round of the 2015 Major League Baseball Draft. In 2016 Kalish was an M...

1964 studio album by Liza MinnelliLiza! Liza!Studio album by Liza MinnelliReleasedOctober 12, 1964RecordedJune 1964StudioCapitol, New York CityGenrePop, vocal, traditionalLabelCapitolProducerSimon RadyLiza Minnelli chronology Liza! Liza!(1964) It Amazes Me(1965) Liza! Liza! is the debut studio album by American singer Liza Minnelli. It was released on October 12, 1964, by Capitol Records. The album contains her interpretations of twelve pop standards. It was recorded in June 1964 at C...

American rock and roll pioneer (1938–1960) Eddie CochranCochran in 1957Background informationBirth nameRay Edward CochranBorn(1938-10-03)October 3, 1938Albert Lea, Minnesota, U.S.DiedApril 17, 1960(1960-04-17) (aged 21)Bath, Somerset, EnglandGenres Rock and roll rockabilly country rhythm and blues Occupation(s)MusiciansongwriterInstrument(s) Guitar piano bass drums vocals Years active1950–1960Labels Ekko Crest Liberty London Musical artist Ray Edward Cochran (/ˈkɒkrən/ KOK-rən; O...

This article is about the local government area. For other uses, see Barossa (disambiguation). Local government area in South AustraliaBarossa District CouncilSouth AustraliaThe location of the Barossa Council in bluePopulation25,066 (LGA 2021)[1] • Density27,48/km2 (7,120/sq mi)Established1996Area912 km2 (352.1 sq mi)MayorMichael Bim Lange [2]Council seatNuriootpaRegionBarossa Light and Lower North[3]State electorate(s)SchubertFederal ...

Surface-mount packaging that uses an array of solder balls This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Ball grid array – news · newspapers · books · scholar · JSTOR (September 2010) (Learn how and when to remove this message) A grid array of solder balls on a printed circuit board after removal of an int...

Questa voce sull'argomento cestisti finlandesi è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Veikko VainioNazionalità Finlandia Altezza205 cm Peso100 kg Pallacanestro RuoloCentro Termine carriera1974 CarrieraGiovanili 1967-1971 BYU Cougars Squadre di club 1964-1967 KTP-Basket1971-1974 Pantterit Nazionale 1965-1973 Finlandia68 (414) Il simbolo → indica un trasferimento in presti...

Model of human interaction proposed in 1968 Drama triangle proposed by the psychiatrist Stephen B. Karpman The Karpman drama triangle is a social model of human interaction proposed by San Francisco psychiatrist Stephen B. Karpman in 1968. The triangle maps a type of destructive interaction that can occur among people in conflict.[1] The drama triangle model is a tool used in psychotherapy, specifically transactional analysis. The triangle of actors in the drama are persecutors, victi...

For the headland located in Newfoundland, see Cape Ray. For the town, see Cape Ray, Newfoundland and Labrador. MV Cape Ray (T-AKR-9679) in 2014 History United States NameCape Ray OwnerMaritime Administration (MARAD)[1] BuilderKawasaki Heavy Industries Ltd., Japan[1][2] Acquired17 Dec 1994[2] Identification IMO number: 7530810 MMSI number: 366841000 Callsign: KAFI General characteristics Class and typeMV Cape Rise (T-AKR-9678) Displacement32,054 t...

Conscription1780 caricature of a press gang Related concepts Alternative civilian serviceCivil conscriptionConscientious objectorConscription crisisCounter-recruitmentDraft-card burningDraft evasionImpressmentLevée en masseMilitary serviceNational servicePenal military unitWar resister By historical country Ottoman EmpireRussian EmpireSoviet Union By modern country ArgentinaAustraliaAzerbaijanBermudaBrazilCanadaChinaCongo-Kinshasa (child soldiers)CubaCyprus (reduction)DenmarkEgyptEritreaFinl...

Aircraft wing configuration with bend at root For other uses, see Gull-wing. DFS Habicht glider showing gull wing profile. Laughing gull showing the wing shape emulated in gull wing aircraft. The gull wing, also known as Polish wing or Puławski wing, is an aircraft wing configuration with a prominent bend in the wing inner section towards the wing root. Its name is derived from the seabirds which it resembles and from the Polish aircraft designer Zygmunt Puławski who started using this desi...




