Inhibitor GABA transaminaze
|
Read other articles:

Untuk pemimpin hoki es Cekoslowakia, lihat Josef Mikoláš. Mikolas JosefJosef pada tahun 2018LahirMikoláš Josef4 Oktober 1995 (umur 28)Praha, Republik CekoTempat tinggalWina, Austria[1]Tahun aktif2013–sekarangKota asalKamenice, Republik Ceko[2]Karier musikGenrePophousefoklorhip hopR&BInstrumenVokalgitarLabelSonyRCASitus webmikolasjosef.com Mikoláš Josef (pelafalan Ceko: ['mikolaːʃ 'jozef]; lahir 4 Oktober 1995), yang lebih dikenal sebagai Mikol...

Kapal perusak Jepang Hatsuyuki Sejarah Kekaisaran Jepang Nama HatsuyukiDipesan 1923 (Tahun Fiskal)Pembangun Arsenal Angkatan Laut MaizuruNomor galangan Perusak No.37Pasang lunas 12 April 1927Diluncurkan 29 September 1928Mulai berlayar 30 Maret 1929Dicoret 15 Oktober 1943Identifikasi Nomor lambung: 11Nasib Tenggelam akibat serangan udara pada 17 Juli 1943 Ciri-ciri umum Kelas dan jenis Kapal perusak kelas-FubukiBerat benaman 1.680 ton panjang (1.710 t) (standar) 1.980 ton panjang (2.010&...

334th Rifle Division (August 1941 – November 1945)Maj. Gen. Nikolai Mikhailovich MishchenkoActive1941–1945Country Soviet UnionBranch Red ArmyTypeDivisionRoleInfantryEngagementsBattle of MoscowToropets-Kholm OffensiveBattle of Smolensk (1943)Dukhovshchina–Demidov OffensiveOperation BagrationBaltic OffensiveRiga OffensiveVistula-Oder OffensiveBattle of KönigsbergDecorations Order of Suvorov 2nd classBattle honoursVitebskCommandersNotablecommandersMaj. Gen. Nikolai Mikhailo...

Group B of the 2015 Copa América was one of the three groups of competing nations in the 2015 Copa América. It consisted of Argentina, Uruguay (title holders), Paraguay, and guests Jamaica of CONCACAF.[1] Group play began on 13 June 2015 and ended on 20 June 2015. Argentina, Paraguay and Uruguay advanced to the quarter-finals. Teams Draw position Team Appearance Previous bestperformance FIFA Rankings October 2014[nb 1] Start of event B1 Argentina 40th Winners (1921, 19...

TecumsehPotret Tecumseh dari sekitar tahun 1868LahirMaret 1768Di tepi Sungai Scioto, didekat Chillicothe, Ohio(lokasi tidak diketahui, lihat Kehidupan Awal)Meninggal5 Oktober 1813 (usia 45)Moravian of the Thames(yang sekarang Chatham-Kent, Ontario)MakamWalpole Island, KanadaKebangsaanShawneeNama lainTecumtha, TekamthiDikenal atasPerang 1812 Pengepungan Detroit Pertempuran Thames † Orang tuaPuckshinwa, Methoataske Tecumseh (Maret 1768 – 5 Oktober, 1813), alias Tecumtha atau Tek...

Лукьяновская тюрьма Местоположение Лукьяновка (Киев), ул. Дегтяревская 13 Координаты 50°27′44″ с. ш. 30°28′02″ в. д.HGЯO Открытие 1863 Медиафайлы на Викискладе Лукьяновская тюрьма (Лукьяновский тюремный замок, Лукьяновский СИЗО, Лукьяновка, памятник архитектуры)...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Taylorcraft Aircraft – news · newspapers · books · scholar · JSTOR (March 2008) (Learn how and when to remove this template message) Taylorcraft AviationCompany typeSubsidiaryIndustryGeneral aviationFounded1935; 89 years ago (1935) in Bradford...

American foundation supporting chemistry research and education The Camille and Henry Dreyfus FoundationFormation1946FounderCamille DreyfusHeadquartersNew York, NY, United StatesPresidentHenry C. WalterRevenue (2015) $2,895,924[1]Expenses (2015)$5,659,652[1]Websitewww.dreyfus.org The Camille and Henry Dreyfus Foundation is a New York City-based foundation founded in 1946 by chemist and investor Camille Dreyfus in honour of his brother, Henry Dreyfus.[2] The two men inv...
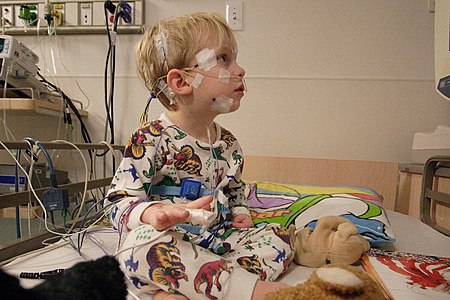
Seorang pasien anak di Rumah Sakit Anak-Anak Saint Louis, 2006 Pasien atau pesakit[1] adalah seseorang yang menerima perawatan medis.[2] Sering kali, pasien menderita penyakit atau cedera dan memerlukan bantuan dokter untuk memulihkannya. Asal mula kata Kata pasien dari bahasa Indonesia analog dengan kata patient dari bahasa Inggris yang artinya sabar. Patient diturunkan dari bahasa Latin yaitu patiens yang memiliki kesamaan arti dengan kata kerja pati yang artinya menderita. ...

Jembatan kayu dengan seorang pekerja sibuk melakukan konsolidasi, di Laos Sebuah jembatan pejalan kaki di Distrik Shaharah, Yaman Jembatan pejalan kaki (disebut juga jembatan penyeberangan, jembatan penyeberangan pejalan kaki, atau penyeberangan pejalan kaki) adalah jembatan yang dirancang khusus untuk pejalan kaki.[1] Sementara arti utama jembatan adalah struktur yang menghubungkan dua titik pada ketinggian di atas tanah, jembatan pejalan kaki juga bisa berupa struktur yang lebih ren...

Cet article est une ébauche concernant une localité autrichienne. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Sibratsgfäll Héraldique Administration Pays Autriche Land Vorarlberg District(Bezirk) Brégence Conseillers municipaux 9 Maire Konrad Stadelmann Code postal A-6952 (Autriche) Immatriculation B Code Commune 80237 Démographie Population 401 hab. (1er janvier 2018[1]) Densité 14 hab./km2...

Keuskupan Vallo della LucaniaDioecesis Vallensis in LucaniaKatolik San Pantaleone church [it]LokasiNegaraItaliaProvinsi gerejawiSalerno-Campagna-AcernoStatistikLuas1.562 km2 (603 sq mi)Populasi- Total- Katolik(per 2010)161.000157,000 (97.5%)Paroki138InformasiDenominasiGereja KatolikRitusRitus RomaPendirianAbad ke-12KatedralCattedrale di S. PantaleoneKepemimpinan kiniPausFransiskusUskupCiro MinieroEmeritusGiuseppe Rocco FavaleSitus webwww.diocesi...

У этого термина существуют и другие значения, см. Западный округ. Западный внутригородской округ город Краснодар Дата основания 1936 год Дата упразднения 1994 Прежние имена Кагановичский, Ленинский районы Микрорайоны Дубинка, Черёмушки, Покровка Площадь 22[1] км² Насе...
2020年夏季奥林匹克运动会波兰代表團波兰国旗IOC編碼POLNOC波蘭奧林匹克委員會網站olimpijski.pl(英文)(波兰文)2020年夏季奥林匹克运动会(東京)2021年7月23日至8月8日(受2019冠状病毒病疫情影响推迟,但仍保留原定名称)運動員206參賽項目24个大项旗手开幕式:帕维尔·科热尼奥夫斯基(游泳)和马娅·沃什乔夫斯卡(自行车)[1]闭幕式:卡罗利娜·纳亚(皮划艇)&#...

يو إف سي على فوكس: هولم ضد شيفتشينكو الجهة المنظمة يو إف سي الرياضة فنون القتال المختلطة البلد الولايات المتحدة يو إف سي ليلة القتال: ماكدونالد ضد لينيكر يو إف سي 201 تعديل مصدري - تعديل يو إف سي على فوكس: هولم ضد شيفتشينكو (بالإنجليزية: UFC on Fox: Holm vs. Shevchenko) �...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Modern School New Delhi – news · newspapers · books · scholar · JSTOR (April 2020) (Learn how and when to remove this message) Private school in New Delhi, Delhi, IndiaModern School, Barakhamba Road, New DelhiAddressBarakhamba RoadNew Delhi, Delhi, 110 001...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Desember 2022. Berikut adalah daftar negara Afrika menurut jumlah penduduk, yang diurutkan berdasarkan proyeksi demografi yang dinormalisasi dari sensus atau data demografi terbaru yang tersedia. Afrika menjadi wilayah dengan pertumbuhan penduduk tercepat di dunia.&...

Linhagem P.2 Variante da SARS-CoV-2 vírus responsável pela COVID-19 e sua pandemia Nome científico Linhagem P.2 Apelido Variante Zeta Primeira detecção em Brasil Variante de Preocupação (VOC)? Não Variante de Interesse (VOI)? Sim Resistencia á vacinação Sem dados claros A linhagem P.2, também conhecida como a variante Zeta, é uma variante do SARS-CoV-2, o vírus que causa o COVID-19. Foi detectado pela primeira vez no estado do Rio de Janeiro, ele contém a mutação E484K...

Painting by Peter Paul Rubens This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (April 2024) (Learn how and when to remove this message) You can help expand this article with text translated from the corresponding article in German. (August 2013) Click [show] for important translation instructions. View a machine-translated version of the German article. Ma...

Schenectady Open 1989Sport Tennis Data17 luglio– 23 luglio (uomini)24 luglio – 30 luglio (donne) Edizione3a SuperficieCemento CampioniSingolare maschile Simon Youl Singolare femminile Laura Gildemeister Doppio maschile Scott Davis / Broderick Dyke Doppio femminile Michelle Jaggard / Hu Na 1988 1990 Lo Schenectady Open 1989 è stato un torneo di tennis giocato sul cemento. È stata la 3ª edizione del torneo, che fa parte del Nabisco Grand Prix 1989 e della categoria Tier V nell'ambito del...