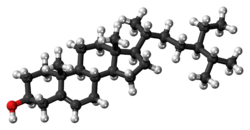Β-Sitosterol
| |||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

1945 film by Emilio Fernández La perlaU.S. theatrical release posterDirected byEmilio FernándezScreenplay byJohn SteinbeckEmilio FernándezJack WagnerBased onThe Pearlby John SteinbeckProduced byOscar DancigersStarringPedro ArmendárizMaría Elena MarquésFernando WagnerCharles RoonerCinematographyGabriel FigueroaEdited byGloria SchoemannMusic byAntonio Díaz CondeProductioncompaniesÁguila FilmsRKO Radio PicturesFilm Asociados Mexico-AmericanosDistributed byRKO Radio PicturesPelículas Mex...

Camellia 4Album studio karya Ebiet G. AdeDirilis1980Direkam1980GenrePop, country, akustikLabelJackson RecordsProduserJackson AriefKronologi Ebiet G. Ade Camellia III (1980)Camellia III1980 Camellia 4 (1980) Langkah Berikutnya (1982)String Module Error: Match not found1982 Camellia 4 adalah album keempat yang dikeluarkan oleh Ebiet G. Ade dari perusahaan rekam Jackson Records yang dirilis pada 26 Desember 1980. Ini adalah album terakhir Ebiet yang menggunakan nama Camellia. Album ini menga...

Basilika Rosario SuciBasilika Minor Rosario SuciBasilika Rosario SuciLokasiTucumánNegara ArgentinaDenominasiGereja Katolik RomaArsitekturStatusBasilika minorStatus fungsionalAktif Basilika Rosario Suci adalah sebuah gereja basilika minor Katolik yang terletak di Tucumán, Argentina. Basilika ini ditetapkan statusnya pada 1941 dan didedikasikan kepada Rosario Suci.[1] Lihat juga Gereja Katolik Roma Gereja Katolik di Argentina Daftar basilika Daftar basilika di Argentina Referensi...

1966 French filmLa Grande VadrouilleFrench theatrical release posterDirected byGérard OuryWritten byMarcel JullianGérard OuryDanièle ThompsonGeorges TabetAndré TabetProduced byRobert DorfmannStarringBourvilLouis de Funès Claudio BrookTerry-ThomasCinematographyAndré DomageAlain DouarinouClaude RenoirEdited byAlbert JurgensonMusic byGeorges AuricHector BerliozProductioncompaniesLes Films Corona Rank Organisation La Magie du CinémaDistributed byValoria Films (France) Rank Film Distributo...

Association football club in Saudi Arabia Not to be confused with Qadsia SC. Football clubAl-Qadsiah FCFull nameAl-Qadsiah Saudi Football ClubNickname(s)Fares Al Sharqiya (Knight of the East)Fakhr Al Sharqiya (Pride of the Eastern Province)Founded1967; 57 years ago (1967)GroundPrince Saud bin Jalawi StadiumKhobar, Saudi ArabiaCapacity11,000[1]OwnerSaudi AramcoChairmanAhmed GhodranManagerMíchelLeagueFirst Division League2022–23FDL, 11th of 18WebsiteClub website Hom...

American film production company KatzSmith ProductionsCompany typeProduction companyIndustryMotion pictures Television showFounded2011Founder Seth Grahame-Smith David Katzenberg HeadquartersUnited StatesKey peopleSeth Grahame-SmithDavid Katzenberg KatzSmith Productions is an American film and television production company, founded by Seth Grahame-Smith and David Katzenberg.[1] The company is known for producing the 2017 horror film It.[2] Overview In 2011, Seth Grahame-Smith a...

Coppa Libertadores 2003 Competizione Coppa Libertadores Sport Calcio Edizione 44ª Organizzatore CONMEBOL Date 22 ottobre 2002 - 2 luglio 2003 Partecipanti 34 (4 alle qualificazioni) Risultati Vincitore Boca Juniors(5º titolo) Secondo Santos Semi-finalisti Independiente Medellín América Statistiche Miglior marcatore Marcelo Delgado (9 gol) Ricardo Oliveira (9 gol) Incontri disputati 138 Gol segnati 406 (2,94 per incontro) Cronologia della competizione 2002...

English rock band (1960–1970) This article is about the band. For their eponymous album, see The Beatles (album). For other uses, see Beatles (disambiguation). Beatle and Fab Four redirect here. For the insect, see Beetle. For other uses, see Fab Four (disambiguation). The BeatlesThe Beatles in 1964; clockwise from top left: John Lennon, Paul McCartney, Ringo Starr and George HarrisonBackground informationOriginLiverpool, EnglandGenresRockpopbeatpsychedeliaYears active1960–1970LabelsParlo...

Head of the judiciary of England and Wales Lord Chief Justice and Lady Chief Justice redirect here. For other uses, see Lord Chief Justice (disambiguation). Lady Chief Justice of England and WalesThe Judiciary of England and WalesIncumbentThe Baroness Carr of Walton-on-the-Hillsince 1 October 2023StyleThe Right HonourableNominatorJudicial Appointments CommissionAppointerMonarch of the United Kingdom,on the recommendation of the Prime Minister and Lord Chancellor[1]Formation29 Nov...

Stasiun Ginan岐南駅Stasiun Ginan, September 2009Lokasi4 Chome Shimoinjiki, Ginan-chō, Hashima-gun, Gifu-ken 501-6018JepangKoordinat35°23′22″N 136°46′08″E / 35.3895°N 136.7689°E / 35.3895; 136.7689Koordinat: 35°23′22″N 136°46′08″E / 35.3895°N 136.7689°E / 35.3895; 136.7689Operator MeitetsuJalur■Jalur Utama Meitetsu NagoyaLetak91.5 km dari ToyohashiJumlah peron2 peron sampingInformasi lainStatusTanpa stafKode sta...

此條目可参照英語維基百科相應條目来扩充。 (2021年5月6日)若您熟悉来源语言和主题,请协助参考外语维基百科扩充条目。请勿直接提交机械翻译,也不要翻译不可靠、低品质内容。依版权协议,译文需在编辑摘要注明来源,或于讨论页顶部标记{{Translated page}}标签。 约翰斯顿环礁Kalama Atoll 美國本土外小島嶼 Johnston Atoll 旗幟颂歌:《星條旗》The Star-Spangled Banner約翰斯頓環礁�...

Polish pole vaulter Not to be confused with Polish footballer Przemysław Czerwiński. Przemysław Czerwiński Przemysław Czerwiński in 2015 Medal record Men's athletics Representing Poland European Championships 2010 Barcelona Pole vault European Team Championships 2010 Bergen Pole vault Przemysław Czerwiński, 2010 Polish Championships in Athletics (Bielsko-Biała) Przemysław Czerwiński (born 28 July 1983) is a Polish pole vaulter. Czerwiński was born in Piła. He finished 5th i...

1900年美國總統選舉 ← 1896 1900年11月6日 1904 → 447張選舉人票獲勝需224張選舉人票投票率73.2%[1] ▼ 6.1 % 获提名人 威廉·麥金利 威廉·詹寧斯·布賴恩 政党 共和黨 民主党 家鄉州 俄亥俄州 內布拉斯加州 竞选搭档 西奧多·羅斯福 阿德萊·史蒂文森一世 选举人票 292 155 胜出州/省 28 17 民選得票 7,228,864 6,370,932 得票率 51.6% 45.5% 總統選舉結果地圖,紅色代表�...

Municipality in Andalusia, SpainHiguera de la SierraMunicipality FlagCoat of armsHiguera de la SierraLocation in SpainCoordinates: 37°50′N 6°26′W / 37.833°N 6.433°W / 37.833; -6.433CountrySpainAutonomous communityAndalusiaProvinceHuelvaComarcaSierra de HuelvaArea • Total24 km2 (9 sq mi)Elevation620 m (2,030 ft)Population (2018)[1] • Total1,293 • Density54/km2 (140/sq mi)DemonymHiguer...

Period in Finnish historyYou can help expand this article with text translated from the corresponding article in Finnish. Click [show] for important translation instructions. Machine translation, like DeepL or Google Translate, is a useful starting point for translations, but translators must revise errors as necessary and confirm that the translation is accurate, rather than simply copy-pasting machine-translated text into the English Wikipedia. Consider adding a topic to this template: ther...

本條目存在以下問題,請協助改善本條目或在討論頁針對議題發表看法。 此條目没有列出任何参考或来源。 (2020年3月11日)維基百科所有的內容都應該可供查證。请协助補充可靠来源以改善这篇条目。无法查证的內容可能會因為異議提出而被移除。 此條目體裁或許更宜作散文而非列表。 (2020年10月25日)如有餘力,请协助将此条目改写为散文。查看编辑帮助。 國立新豐高級中�...

Diacritical mark Not to be confused with overline or bar (diacritic). O macron, O-, and Ū redirect here. For the Greek letter, see omicron. For the o- prefix in Japanese, see Japanese honorifics. For the Indic vowel, see Ū (Indic). ◌̄MacronU+0304 ◌̄ COMBINING MACRONSee alsoU+0331 ◌̱ COMBINING MACRON BELOW A macron (/ˈmækrɒn, ˈmeɪ-/ MAK-ron, MAY-) is a diacritical mark: it is a straight bar ¯ placed above a letter, usually a vowel. Its name derives fro...

American actress (born 1960) Melissa LeoLeo at the 2009 Tribeca Film FestivalBorn (1960-09-14) September 14, 1960 (age 63)New York City, New York, United StatesEducationState University of New York, PurchaseOccupationActressYears active1984–presentPartnerJohn Heard (1986–1988)[1]Children1RelativesChristine Leo Roussel (aunt) Melissa Chessington Leo (born September 14, 1960)[2] is an American actress. She is the recipient of several accolades, including an Academy...
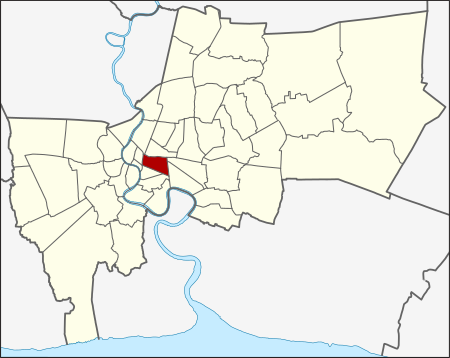
ปทุมวัน (بالتايلندية: เขตปทุมวัน) خريطة الموقع الشعار (بالتايلندية: บูชาท้าวมหาพรหม ชื่นชมวังสมเด็จย่า ศูนย์การค้ามากมี ลุมพินีสวนสาธารณะ ศิลปะมวยไทย สถานีรถไฟหัวลำโพง เชื่อมโยงรถไฟฟ้า ส...

Questa voce sugli argomenti evoluzione e paleontologia è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Un'illustrazione raffigurante diverse forme di vita apparse nel corso dell'esplosione cambriana. L'esplosione cambriana, conosciuta anche come esplosione del Cambriano o informalmente in inglese come Biological Big Bang (in italiano, Big Bang biologico), fu un evento nella storia della Terra consistente nell'improvvisa comparsa della maggioranza dei ...