Omadacycline
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:
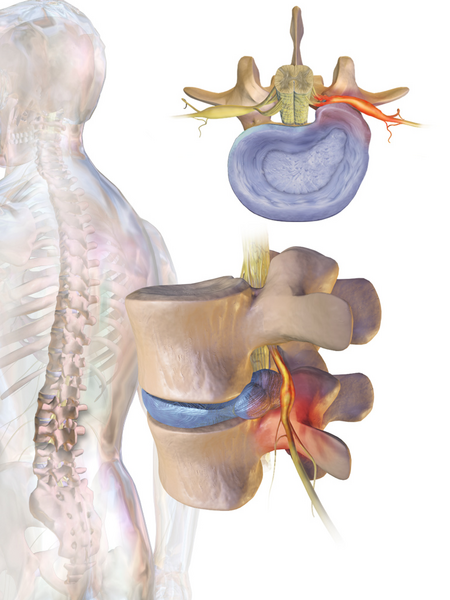
Le informazioni riportate non sono consigli medici e potrebbero non essere accurate. I contenuti hanno solo fine illustrativo e non sostituiscono il parere medico: leggi le avvertenze. RadicolopatiaLe vertebre C5-C6, seguite da C6-C7, sono la più comune localizzazione delle radicolopatie cervicali-dorsali del colloSpecialitàneurochirurgia Classificazione e risorse esterne (EN)ICD-9-CM723.4, 724.4 e 729.2 MeSHD011843 Modifica dati su Wikidata · Manuale La radicolopatia o neuropatia ra...

Hendra PurnamaInformasi pribadiNama lengkapHendra PurnamaLahir12 Januari 1997 (umur 27)Bantul, IndonesiaTinggi169 m (554 ft 6 in)Berat64 kg (141 pon) (141 pon) OlahragaNegara IndonesiaOlahragaPanahanLombaRecurve Rekam medali Panahan putra Mewakili Indonesia Pesta Olahraga Asia Tenggara Filipina 2019 Beregu putra Filipina 2019 Perorangan putra Singapura 2015 Beregu putra Diperbarui pada 9 Desember 2019. Hendra Purnama (lahir 12 Januari 1997) adalah...

Asam fluoroasetat Nama Nama IUPAC (preferensi) Asam fluoroasetat Nama lain Asam 2-fluoroasetatAsam monofluoroasetatMonofluoroasetatAsam fluoroetanoatAsam simonat Penanda Nomor CAS 144-49-0 Y Model 3D (JSmol) Gambar interaktif 3DMet {{{3DMet}}} Referensi Beilstein 1739053 ChEBI CHEBI:30775 ChEMBL ChEMBL509273 ChemSpider 10205670 Nomor EC Referensi Gmelin 25730 KEGG C06108 PubChem CID 5237 Nomor RTECS {{{value}}} UNII AP1JV9U41M Y Nomor UN 2642 CompTox Dashboard (EPA) DTXSID0041981 I...

Chemical compound DieticyclidineClinical dataATC codenoneLegal statusLegal status CA: Schedule I UK: Class B Identifiers IUPAC name N,N-diethyl-1-phenylcyclohexan-1-amine CAS Number2201-19-6 YPubChem CID604690ChemSpider525655 NUNII8JY6P22UVDCompTox Dashboard (EPA)DTXSID00944617 Chemical and physical dataFormulaC16H25NMolar mass231.383 g·mol−13D model (JSmol)Interactive image SMILES CCN(CC)C1(CCCCC1)C2=CC=CC=C2 InChI InChI=1S/C16H25N/c1-3-17(4-2)16(13-9-6-10-14-16)1...
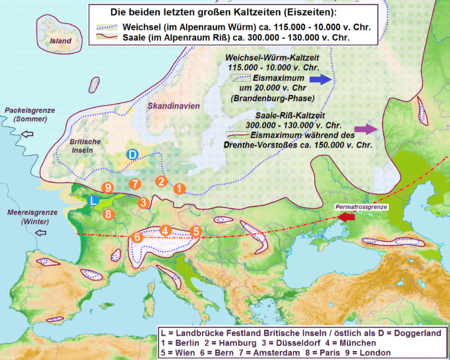
Last glacial period in the Alpine region This article needs to be updated. Please help update this article to reflect recent events or newly available information. Last update: 2008 chart and 1972 dating when it should be 2013 iugs chart and 2012 walker paper (see Holocene talk page) (May 2016) Violet: The extent of the Alpine ice sheet in the Würm glaciation. Blue: The extent in earlier ice ages The Würm glaciation or Würm stage (German: Würm-Kaltzeit or Würm-Glazial, colloquially often...

LopudView from Sutvrač FortLopudGeographyLocationAdriatic SeaCoordinates42°41′N 17°57′E / 42.683°N 17.950°E / 42.683; 17.950ArchipelagoElaphiti IslandsArea4.63 km2 (1.79 sq mi)Highest elevation214 m (702 ft)Highest pointPolačicaAdministrationCroatiaCountyDubrovnik-NeretvaDemographicsPopulation278 (2021) Lopud (pronounced [lɔ̌pud]) is a small island off the coast of Dalmatia, southern Croatia. Lopud is economically...

Japanese ship Not to be confused with Tōya Maru. This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. Please help improve this article by introducing more precise citations. (July 2014) (Learn how and when to remove this template message) Toyama Maru Toyama Maru in 1941 History Japan NameToyama Maru Laid down4 August 1913 Launched20 March 1915 Completed3 June 1915 FateSunk by USS Sturgeon near T...

AtiWanita Ati di PanayJumlah populasiperkiraan 2,000+ (1980: 1,500 penutur Ati)[1]Daerah dengan populasi signifikan FilipinaVisayasBahasaAti, Aklanon/Malaynon, Hiligaynon, Kinaray-a, Filipino, InggrisAgamaAnimisme, Kekristenan (Katolik Roma)Kelompok etnik terkaitNegrito, Bisaya Suku Ati adalah suku bangsa Negrito di Visayas di Filipina Tengah. Jumlah mereka tersebar di Pulau Boracay, Panay, dan Negros. Secara genetik, mereka identik[2] dengan suku Negrito lain seperti Suk...

1st episode of the 1st season of Batman Hi Diddle RiddleBatman episodeEpisode no.Season 1Episode 1Directed byRobert ButlerWritten byLorenzo Semple Jr.Production code6028-Pt. 1Original air dateJanuary 12, 1966 (1966-01-12)Guest appearancesMichael Fox Jack Barry Ben Astar, Damian O'Flynn William Dozier, Jill St. John Richard Reeves (uncredited)Special Guest Villain: Frank Gorshin as The RiddlerEpisode chronology ← Previous— Next →Smack in the Middle List ...

Pour les articles homonymes, voir La Ville fantôme et Ghost Town. Bodie, ville fantôme du sud-est californien. Une ville fantôme est une ville initialement habitée et animée qui a été abandonnée. Généralement, l'abandon d'une ville résulte du tarissement de l'activité économique qui la faisait vivre ou d'une catastrophe d'origine naturelle (désertification, inondation, séisme, éruption volcanique, etc.) ou humaine (bataille, crise économique, accident industriel, désaf...

Part of a series onBritish law Acts of Parliament of the United Kingdom Year 1801 1802 1803 1804 1805 1806 1807 1808 1809 1810 1811 1812 1813 1814 1815 1816 1817 1818 1819 1820 1821 1822 1823 1824 1825 1826 1827 1828 1829 1830 1831 1832 1833 1834 1835 1836 1837 1838 1839 1840 1841 1842 1843 1844 1845 1846 1847 1848 1849 1850 1851 1852 1853 1854 1855 1856 1857 1858 1859 1860 1861 1862 1863 1864 1865 1866 1867 1868 1869 1870 1871 1872 1873 1874 1875 1876 1877 1878 ...

McLaren P1Descrizione generaleCostruttore McLaren Automotive Tipo principaleCoupé Produzionedal 2013 al 2015 Sostituisce laMcLaren F1 Sostituita daMcLaren Senna Esemplari prodotti381 (di cui 6 LM)[senza fonte] Altre caratteristicheDimensioni e massaLunghezza4588 mm Larghezza1946 mm Altezza1188 mm Passo2680 mm Massa1610 kg AltroAssemblaggioWoking, Surrey (Inghilterra) ProgettoPaul Mackenzie StileFrank Stephenson[1] Stessa famigliaMcLaren...

Approach to feminism influenced by ecologist movement French writer Françoise d'Eaubonne coined the term in a 1974 book Part of a series onGreen politics Core topics Climate change litigation Fossil fuels lobby Green politics Green party List of topics Politics of climate change Four pillars Ecological wisdom Social justice Grassroots democracy Nonviolence Perspectives Alter-globalization Bright green environmentalism Criticisms of globalization Deep ecology Degrowth Dirty hands Disinvestmen...

كيج هيشت معلومات شخصية الميلاد 18 فبراير 1998 (26 سنة)[1] الجنسية الولايات المتحدة الحياة العملية الفرق رالي سايكلينغ (2022–) المهنة دراج نوع السباق سباق الدراجات الهوائية تعديل مصدري - تعديل كيج هيشت (بالإنجليزية: Gage Hecht) هو دراج أمريكي، ولد في 18 فبرا�...

Sporting event delegationGreece at the2022 Winter OlympicsFlag of GreeceIOC codeGRENOCHellenic Olympic CommitteeWebsitewww.hoc.grin Beijing, China4–20 February 2022Competitors5 (2 men and 3 women) in 2 sportsFlag bearers (opening)Apostolos AngelisMaria NtanouFlag bearer (closing)Ioannis AntoniouMedals Gold 0 Silver 0 Bronze 0 Total 0 Winter Olympics appearances (overview)193619481952195619601964196819721976198019841988199219941998200220062010201420182022 Greece competed ...

Election 1950 Wisconsin gubernatorial election ← 1948 November 7, 1950 1952 → Nominee Walter J. Kohler Jr. Carl W. Thompson Party Republican Democratic Popular vote 605,649 525,319 Percentage 53.21% 46.16% County resultsKohler: 50–60% 60–70% 70–80%Thompson: 50–60% Governor before election Oscar Rennebohm Republican Elected Governor W...

American cable company This article relies excessively on references to primary sources. Please improve this article by adding secondary or tertiary sources. Find sources: Cable One – news · newspapers · books · scholar · JSTOR (August 2020) (Learn how and when to remove this message) Cable One, Inc.Company typePublicTraded asNYSE: CABOS&P 600 componentIndustryCable TV, broadband phone, Internet, Fiber ServicesFounded1986; 38 year...
ليث بن أبي سليم معلومات شخصية اسم الولادة لَيْث بن أَبي سُلَيْم بن زنيم الكنية أَبُو بَكْر اللقب القرشي الحياة العملية الطبقة الطبقة الخامسة، صغار التابعين روى له روى له مُسْلِم مقرونًا بأبي إِسْحَاق الشيباني، وروى له الباقون المهنة مُحَدِّث تعديل مصدري - تعديل لَ...

President of Kyrgyzstan from 2010 to 2011 Roza OtunbayevaРоза ОтунбаеваOtunbayeva in 20113rd President of KyrgyzstanIn office3 July 2010 – 1 December 2011Acting: 7 April 2010 – 3 July 2010Prime MinisterAlmazbek AtambayevOmurbek Babanov (Acting)Almazbek AtambayevPreceded byKurmanbek BakiyevSucceeded byAlmazbek AtambayevMinister of Foreign AffairsIn office26 February 1992 – 10 October 1992Prime MinisterTursunbek ChyngyshevPreceded byMuratbek ImanaliyevSuccee...

Term for a hypothetical homogeneous pre-Indo-European culture This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (April 2009) (Learn how and when to remove this message) This article possibly contains original r...
