HSAB theory
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Piala Asia U-20 AFC 20232023 yil U-20 Osiyo chempionatiInformasi turnamenTuan rumah UzbekistanJadwalpenyelenggaraan1–18 Maret 2023Jumlahtim peserta16 (dari 1 konfederasi)Tempatpenyelenggaraan2 (di 2 kota)Hasil turnamenJuara Uzbekistan (gelar ke-1)Tempat kedua IrakStatistik turnamenJumlahpertandingan31Jumlah gol69 (2,23 per pertandingan)Jumlahpenonton169.342 (5.463 per pertandingan)Pencetak golterbanyak Naoki Kumata(5 gol)← 20202018 2025 → Selur...

Topan dan AisyahPoster resmiGenre Drama Roman Religi Fantasi PembuatMNC PicturesSkenarioAviv ElhamSutradaraIip S. HananPemeran Syakir Daulay Tissa Biani Imelda Therine Firda Razak Athalla Naufal Penggubah lagu temaArmadaLagu pembukaPergi Pagi Pulang Pagi oleh ArmadaLagu penutupPergi Pagi Pulang Pagi oleh ArmadaPenata musikJoseph S. DjafarNegara asalIndonesiaBahasa asliBahasa IndonesiaJmlh. musim1Jmlh. episode16 (daftar episode)ProduksiProduser eksekutif Filriady Kusmara Rista Ferina Pr...

Central Bank of Morocco Bank Al-Maghribبنك المغربHeadquartersRabat, MoroccoCoordinates34°01′10″N 6°50′09″W / 34.01944°N 6.83583°W / 34.01944; -6.83583Ownership100% state ownership[1]GovernorAbdellatif Jouahri[2]Central bank ofMoroccoCurrencyMoroccan dirhamMAD (ISO 4217)Reserves21 390 million USD[1]Websitewww.bkam.ma Monogram of Bank al-Maghrib on its head office building in Rabat The Bank Al-Maghrib (Arabic: �...

Questa voce sull'argomento arbitri di calcio francesi è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Karim Abed Informazioni personali Arbitro di Calcio Sezione Brignoles Attività nazionale Anni Campionato Ruolo 2014-20152015-2016- CFALigue 2Ligue 1 ArbitroArbitroArbitro Karim Abed Abed (Brignoles, 18 dicembre 1988) è un arbitro di calcio francese. Carriera Karim Abed debutta in massima serie francese come arbitro nella stagione 2016-2017[1]...

Matthew Spiranovic Spiranovic pada tahun 2013Informasi pribadiNama lengkap Matthew Thomas Spiranovic[1]Tanggal lahir 27 Juni 1988 (umur 35)Tempat lahir Geelong, Victoria, AustraliaTinggi 1,93 m (6 ft 4 in)Posisi bermain BekInformasi klubKlub saat ini Western Sydney Wanderers FCNomor 13Karier junior North Geelong Warriors Keilor Park Melbourne Knights2004–2005 VIS2005 Melbourne Victory FC2006 AIS2007 1. FC NürnbergKarier senior*Tahun Tim Tampil (Gol)2004 North G...

American football player (born 1987) American football player Darren McFaddenMcFadden with the Cowboys in 2015No. 20Position:Running backPersonal informationBorn: (1987-08-27) August 27, 1987 (age 36)Little Rock, Arkansas, U.S.Height:6 ft 1 in (1.85 m)Weight:222 lb (101 kg)Career informationHigh school:Oak Grove (North Little Rock, Arkansas)College:Arkansas (2005–2007)NFL draft:2008 / Round: 1 / Pick: 4Career history Oakland Raiders (2008–...

Earth observation satellite Greenhouse Gases Observing SatelliteModel of GOSAT at Tsukuba Space Center Space DomeNamesIbukiMission typeEnvironmentalOperatorJAXACOSPAR ID2009-002A SATCAT no.33492Websiteglobal.jaxa.jp/projects/sat/gosat/index.htmlMission duration5 years (planned)Elapsed: 15 years, 3 months, 8 days Spacecraft propertiesManufacturerMitsubishi ElectricLaunch mass1,750 kilograms (3,860 lb)[1]Power3.8 kilowatts[1] Start of missionLaunch date2...

Bihorel La mairie. Blason Administration Pays France Région Normandie Département Seine-Maritime Arrondissement Rouen Intercommunalité Métropole Rouen Normandie Maire Mandat Pascal Houbron 2020-2026 Code postal 76420 Code commune 76095 Démographie Gentilé Bihorellais Populationmunicipale 8 199 hab. (2021) Densité 3 267 hab./km2 Géographie Coordonnées 49° 27′ 19″ nord, 1° 07′ 01″ est Altitude Min. 80 mMax. 160 m Su...

Северный морской котик Самец Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:Синапси...

土库曼斯坦总统土库曼斯坦国徽土库曼斯坦总统旗現任谢尔达尔·别尔德穆哈梅多夫自2022年3月19日官邸阿什哈巴德总统府(Oguzkhan Presidential Palace)機關所在地阿什哈巴德任命者直接选举任期7年,可连选连任首任萨帕尔穆拉特·尼亚佐夫设立1991年10月27日 土库曼斯坦土库曼斯坦政府与政治 国家政府 土库曼斯坦宪法 国旗 国徽 国歌 立法機關(英语:National Council of Turkmenistan) ...

Частина серії проФілософіяLeft to right: Plato, Kant, Nietzsche, Buddha, Confucius, AverroesПлатонКантНіцшеБуддаКонфуційАверроес Філософи Епістемологи Естетики Етики Логіки Метафізики Соціально-політичні філософи Традиції Аналітична Арістотелівська Африканська Близькосхідна іранська Буддій�...

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Taman Wisata Wendit – berita · surat kabar · buku · cendekiawan · JSTOR Topik artikel ini mungkin tidak memenuhi kriteria kelayakan umum. Harap penuhi kelayakan artikel dengan: menyertakan sumber-sumber ...

Church in Staraya Ladoga, RussiaSt. George's ChurchView from the wall of the fortress59°59′50.60″N 32°17′54.40″E / 59.9973889°N 32.2984444°E / 59.9973889; 32.2984444LocationStaraya LadogaCountryRussiaDenominationRussian OrthodoxHistoryDedicationSaint GeorgeArchitectureStyleRussianCompletedBetween 1180 and 1200 St. George's Church (Russian: Геóргиевская цéрковь, цéрковь Святóго Геóргия) in the selo of Staraya Ladoga, V...
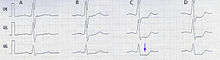
Depression of the ST segment on an electrocardiogram Illustration of upsloping ST segment depression ST depression refers to a finding on an electrocardiogram,[1][2] wherein the trace in the ST segment is abnormally low below the baseline. Causes It is often a sign of myocardial ischemia, of which coronary insufficiency is a major cause. Other ischemic heart diseases causing ST depression include: Subendocardial ischemia[3] or even infarction.[4] Subendocardial...

631 هـمعلومات عامةجزء من تقويم هجري تاريخ البدء 6 أكتوبر 1233[1] تاريخ الانتهاء 25 سبتمبر 1234[1] المواليد قائمة مواليد 631 هـ الوفيات قائمة وفيات 631 هـ لديه جزء أو أجزاء محرم 631 هـصفر 631 هـربيع الأول 631 هـ 630 هـ 632 هـ تعديل - تعديل مصدري - تعديل ويكي بيانات قرن: قرن 6 - قرن 7 - قرن 8 عق�...

Comarca in Galicia, SpainO DezaComarcaCountry SpainAutonomous community GaliciaProvincePontevedraCapitalLalínMunicipalities List Silleda, Vila de Cruces, Agolada, Rodeiro, Dozón, Lalín Area • Total1,024.7 km2 (395.6 sq mi)Population (2018)[1] • Total40,063 • Density39/km2 (100/sq mi)DemonymdezanoTime zoneUTC+1 (CET) • Summer (DST)UTC+2 (CEST) O Deza is a comarca in the northeast corner of the Galicia...

16 BC Roman book by Ovid The Roman poet Ovid, born in the city. Amores (Latin: Amōrēs, lit. 'The Loves')[1] is Ovid's first completed book of poetry, written in elegiac couplets. It was first published in 16 BC in five books, but Ovid, by his own account, later edited it down into the three-book edition that survives today. The book follows the popular model of the erotic elegy, as made famous by figures such as Tibullus or Propertius, but is often subversive and humorous with...

Son of Leon Trotsky, communist writer In this name that follows Eastern Slavic naming customs, the patronymic is Lvovich and the family name is Sedov. Lev SedovBorn(1906-02-24)24 February 1906Died16 February 1938(1938-02-16) (aged 31)Paris, FranceResting placeCimetière parisien de Thiais, ParisParent(s)Leon Trotsky and Natalia SedovaRelativesZinaida Volkova (half-sister) Lev Lvovich Sedov (‹See Tfd›Russian: Лев Львович Седов, also known as Leon Sedov; 24 February ...

Monarchy in Europe (1282–1918) Not to be confused with Habsburg Spain. Habsburg monarchyMonarchia Austriaca (Latin) Habsburgermonarchie (German)1282–1918 Flag of Austria (since 1804)[1] Middle common coat of arms of 1866–1915 The Habsburg monarchy on the eve of the French Revolution, 1789CapitalViennaReligion Roman Catholicism (official)GovernmentMonarchyMonarch • 1282–1291 Rudolf I[a]• 1452–1493 Frederick III[b]• 1508–15...

1990 Manipuri film Ishanou (The Chosen One)Official posterDirected byAribam Syam SharmaWritten byM. K. Binodini DeviProduced byAribam Syam SharmaStarringAnoubam KiranmalaKangabam TombaCinematographyGirish PadhiarEdited byUjjal NandyMusic byAribam Syam SharmaProductioncompanyAribam Syam Sharma ProductionsDistributed byDoordarshan Kendra GuwahatiRelease date 6 July 1990 (1990-07-06) Running time90 minutesCountryIndiaLanguageMeitei language (officially called Manipuri language) Is...






