Electron affinity
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Ibrahima Traoré Informasi pribadiTanggal lahir 21 April 1988 (umur 35)Tempat lahir Villepinte, PrancisTinggi 1,76 m (5 ft 9+1⁄2 in)Posisi bermain GelandangKarier junior2001–2004 Charenton2004–2005 LevalloisKarier senior*Tahun Tim Tampil (Gol)2005–2006 Levallois 4 (0)2006–2009 Hertha BSC II 62 (12)2007–2009 Hertha BSC 1 (0)2009–2011 FC Augsburg 45 (8)2011–2014 VfB Stuttgart 75 (6)2014–2021 Borussia Mönchengladbach 100 (6)Total 287 (32)Tim nasional�...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada November 2022. Braulio ÁvilaInformasi pribadiNama lengkapBraulio Ávila JuárezLahir5 Maret 1986Chiautempan, Tlaxcala OlahragaOlahragaTinju Rekam medali Tinju amatir putra Mewakili Meksiko Pesta Olahraga Amerika Tengah dan Karibia 2006 Cartagena Kelas te...
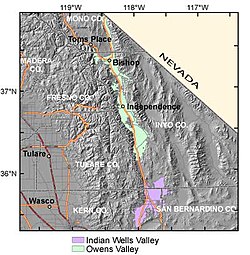
Valley in California, United States Indian Wells ValleyIndian Wells Valley, showing Ridgecrest, California and the China Lake area.Length33 miles (53 km) N-SGeographyBorders onArgus Range (E)Coso Range (N)El Paso Mountains (S)Sierra Nevada (W)Coordinates35°42′10″N 117°41′11″W / 35.70278°N 117.68639°W / 35.70278; -117.68639 Indian Wells Valley is an arid north-south basin in east-central California. In the geologic sense, it is a southern extensio...

Town in Virginia, United States Town in VirginiaHaymarket, VirginiaTownTown of Haymarket SealNickname: The CrossroadsLocation in Prince William County and the state of VirginiaHaymarketShow map of Prince William areaHaymarketShow map of Northern VirginiaHaymarketShow map of VirginiaHaymarketShow map of the United StatesCoordinates: 38°48′46″N 77°38′6″W / 38.81278°N 77.63500°W / 38.81278; -77.63500Country United StatesState VirginiaCountyPrinc...

Komputer Atari Falcon030 beserta monitor Atari Falcon030 Computer System atau Atari Falcon adalah komputer yang diluncurkan oleh Atari Corporation pada tahun 1992.[1] Komputer ini menggunakan Motorola 68030 sebagai CPU utamanya, dan mempunyai digital signal processor Motorola 56000, sebuah prosesor yang membedakan komputer ini dari komputer-komputer lain yang berada di pasaran saat komputer ini diluncurkan. Atari Falcon pada dasarnya adalah versi terakhir dari seri komputer Atari ST. ...

Indian tribe in California, United States Cedarville RancheriaTotal population35 enrolled members,[1]26 rancheria population (2011)[2]Regions with significant populations United States ( California)LanguagesEnglish, Northern PaiuteRelated ethnic groupsother Northern Paiute people The Cedarville Rancheria is a federally recognized tribe of Northern Paiute people in Modoc County, California, about 30 miles (48 km) south of the Oregon border.[2] Cedarville ...

Mayor and Commonalty and Citizens of the City of London City of London CorporationJenisJenisPemerintahan daerah dari City of London PimpinanWali Kota TertinggiNicholas Lyons sejak 11 November 2022 Sekretaris KotaIan Thomas sejak 21 Februari 2023 Anggota100 Common Councilmen25 AldermenPemilihanPemilihan terakhir Court of AldermenBervariasi – mandat individual, sampai 6 tahun masa jabatanPemilihan terakhir Court of Common CouncilMaret 2009 – 4 tahun masa jabatanTempat bersidangGuildhall, Lo...

Bilateral relationsTurkish–British relations United Kingdom Turkey Diplomatic missionEmbassy of United Kingdom, AnkaraEmbassy of Turkey, London The relations between Turkey and the United Kingdom have a long history. The countries have been at war several times, such as within the First World War. They have also been allied several times, such as in the Crimean War. Both countries currently maintain relations via the British Embassy in Ankara[1] and the Turkish Embassy in London.&#...

1930 Mickey Mouse cartoon The Cactus KidDirected byWalt DisneyProduced byWalt DisneyStarringWalt DisneyMarcellite GarnerAnimation byNorm FergusonProductioncompanyWalt Disney StudiosDistributed byColumbia PicturesRelease date May 10, 1930 (1930-05-10)[1] Running time7:25CountryUnited StatesLanguageEnglish The Cactus Kid is a Mickey Mouse short animated film first released on May 10, 1930, as part of the Mickey Mouse film series.[2] It was the eighteenth Mickey Mo...

Untuk kegunaan lain, lihat Sluha Narodu (partai politik). Hamba RakyatGenre Komedi politik PembuatVolodymyr ZelenskyyPemeran Volodymyr Zelenskyy Stanislav Boklan Olena Kravets Jury Krapov Mykhailo Fatalov Oleksandr Pikalov Eugene Koshovyi Viktor Saraykin Natalia Sumska Kateryna Kisten Angelina Zelenskyy Penggubah lagu temaDmytro ShurovPenata musik Andriy Kiryuschenko Negara asalUkrainaBahasa asliRusiaUkrainaJmlh. musim3Jmlh. episode51ProduksiProduser eksekutif Volodymyr Zelenskyy Produs...

Франц Саксен-Кобург-Заальфельдскийнем. Franz von Sachsen-Coburg-Saalfeld герцог Саксен-Кобург-Заальфельдский 8 сентября 1800 — 9 декабря 1806 Предшественник Эрнст Фридрих Саксен-Кобург-Заальфельдский Преемник Эрнст I Саксен-Кобург-Заальфельдский Рождение 15 июля 1750(1750-07-15)Кобург, Сакс...

مستشفى جينينتان إحداثيات 30°40′09″N 114°16′53″E / 30.66929159°N 114.2814184°E / 30.66929159; 114.2814184 معلومات عامة الدولة الصين سنة التأسيس 2008 معلومات أخرى الموقع الإلكتروني الموقع الرسمي رقم الهاتف +86-27-8636-5341[1] تعديل مصدري - تعديل مستشفى جينينتان (بالصينية: 武�...

City in Utah, United States City in Utah, United StatesFairview, UtahCityFairview City Hall, July 2011Location in Sanpete County and the state of Utah.Coordinates: 39°37′44″N 111°26′18″W / 39.62889°N 111.43833°W / 39.62889; -111.43833CountryUnited StatesStateUtahCountySanpeteFounded1859Founded byJames N. JonesGovernment • MayorBrad WelchArea[1] • Total1.26 sq mi (3.26 km2) • Land1.26 sq m...

Sejenis kunai Kunai (苦内 atau 苦無code: ja is deprecated ) adalah peralatan berukuran kecil yang digunakan ninja. Alat ini menyerupai pisau berujung runcing dan sering digunakan seperti kuku oleh para ninja untuk untuk menempel di dinding sewaktu memanjat atau menggali tanah. Kunai berukuran sekitar 10–15 cm sehingga cukup kecil untuk dibawa ke mana-mana dan disembunyikan di balik baju dari penglihatan orang. Alat ini diketahui sebagai senjata standar bagi ninja seperti halnya mak...

Artikel ini membutuhkan rujukan tambahan agar kualitasnya dapat dipastikan. Mohon bantu kami mengembangkan artikel ini dengan cara menambahkan rujukan ke sumber tepercaya. Pernyataan tak bersumber bisa saja dipertentangkan dan dihapus.Cari sumber: Sunset Strip – berita · surat kabar · buku · cendekiawan · JSTOR (June 2006) The Strip terkenal karena iklan dinding-ke-dindingnya Sunset Strip merupakan nama yang diberikan pada sebagian Sunset Boulevard sep...

Terrorist incidents in Syria[1][2] Year Number ofincidents Deaths Injuries 2020 1 46 50 2019 7 107 252 2018 1 258 180 2017 3 294 150 2016 384 2,761 2,830 2015 485 3,916 2,978 2014 328 3,301 1,980 2013 278 1,558 2,237 2012 179 876 1,927 2011 49 163 215 2010 0 0 0 2009 0 0 0 2008 1 18 14 2007 0 0 0 2006 1 5 0 2005 0 0 0 2004 1 4 2 2003 0 0 0 2002 0 0 0 2001 0 0 0 2000 0 0 0 1999 0 0 0 1998 1 0 0 1997 1 2 0 1996 2 15 50 1995 0 0 0 1994 0 0 0 1993 0 0 0 1992 0 0 0 1991 0 0 0 1990...

この項目では、フランスの都市について説明しています。その他の用法については「ヴェルサイユ (曖昧さ回避)」をご覧ください。 Versailles 市章 行政国 フランス地域圏 (Région) イル・ド・フランス地域圏県 (département) イヴリーヌ県(県庁所在地)郡 (arrondissement) ヴェルサイユ郡小郡 (canton) 2小郡庁所在地INSEEコード 78646郵便番号 78000市長(任期) フランソワ・ド・マジ...

千代丸 一樹 基礎情報四股名 木下→千代丸本名 木下 一樹愛称 千代丸たん、マルちゃん、ミスター肉団子、木下兄[1]、ちよまるタン[2]生年月日 (1991-04-17) 1991年4月17日(33歳)出身 鹿児島県志布志市身長 178.0cm体重 166.0kgBMI 52.4所属部屋 九重部屋得意技 突き・押し[3]成績現在の番付 西幕下3枚目最高位 東前頭5枚目生涯戦歴 589勝585敗44休(103場所)幕内戦�...

Capital and largest city in Northern Ireland This article is about the city in Northern Ireland. For other uses, see Belfast (disambiguation). Place in Northern Ireland, United KingdomCity of Belfast Irish: Béal FeirsteUlster Scots: BilfawstCapital city, district, and boroughSkyline and buildings throughout the City of Belfast Coat of armsMotto(s): Latin: Pro Tanto Quid Retribuamus, lit. 'what shall we give in return for so much'Belfast shown within Northern IrelandCo...
Islamic movement Ahmadiyya by country Africa Algeria Angola Benin Botswana Burkina Faso Burundi Cameroon Cape Verde Central African Republic Chad Comoros Côte d'Ivoire DR of Congo Republic of Congo Djibouti Egypt Equatorial Guinea Eritrea Ethiopia Gabon Gambia Ghana Guinea Guinea-Bissau Kenya Lesotho Liberia Libya Madagascar Malawi Mali Mauritania Mauritius Morocco Mozambique Namibia Niger Nigeria Rwanda São Tomé and Príncipe Senegal Seychelles Sierra Leone Somalia South Africa Sudan Swaz...



