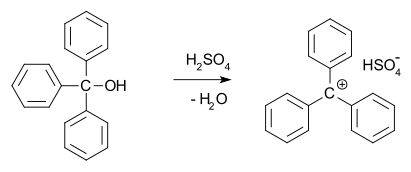
Carbocation
|
Read other articles:

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Dengke mas naniura – berita · surat kabar · buku · cendekiawan · JSTOR Dengke mas naniura adalah hidangan tradisional masyarakat Batak Toba. Dahulu, masakan naniura dikhususkan untuk raja saja, tetapi ka...

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Lokasi paling ekstrem di dunia – berita · surat kabar · buku · cendekiawan · JSTOR Keakuratan artikel ini diragukan dan artikel ini perlu diperiksa ulang dengan mencantumkan referensi yang dapat dipertan...

Cet article concerne la langue japonaise. Pour le peuple japonais, voir Japonais (peuple). Japonais日本語 (nihongo) Pays Japon Nombre de locuteurs environ 128 millions (2020) Nom des locuteurs Japonophones[1], ou Nipponophones Typologie SOV, agglutinante, accusative, morique, à accent de hauteur Écriture Kanjis et kanas (hiraganas et katakanas) Classification par famille - langues japoniques - langues japoniques insulaires - vieux japonais - japonais Statut officiel Langue officiell...

English, Scottish, Irish and Great Britain legislationActs of parliaments of states preceding the United Kingdom Of the Kingdom of EnglandRoyal statutes, etc. issued beforethe development of Parliament 1225–1267 1275–1307 1308–1325 Temp. incert. 1327–1411 1413–1460 1461 1463 1464 1467 1468 1472 1474 1477 1482 1483 1485–1503 1509–1535 1536 1539–1540 1541 1542 1543 1545 1546 1547 1548 1549 1551 1553 1554 1555 &...

Maa BhoomiSutradaraGoutam GhoseProduserB. Narsing RaoG. RavindranathDitulis olehB. Narsing Rao (permainan latar)Krishan Chander (cerita)Partha Banerjee (dialog)PemeranSai ChandRami ReddyPenata musikVinjamuri Seetha DeviSinematograferKamal NaikPenyuntingRajagopalPerusahaanproduksiSaradhi StudiosTanggal rilis 1980 (1980) Durasi158 menitNegaraIndiaBahasaTelugu Maa Bhoomi (Telugu: మా భూమి, Indonesia: Tanah Kami) adalah sebuah film drama Telugu 1980 yang ditulis dan diproduk...

William Odom William Eldridge Odom (lahir 23 Juni 1932) adalah seorang jenderal berbintang 3 purnawirawan Angkatan Darat Amerika Serikat yang pernah menjabat sebagai Direktur Badan Keamanan Nasional di bawah Presiden Ronald Reagan, yang merupakan puncak kariernya selama 31 tahun dalam bidang intelijen militer, khususnya dalam masalah-masalah yang berkaitan dengan Uni Soviet. Setelah pensiunnya dari kemiliteran, ia menjadi pakar kebijakan tangki berpikir dan seorang profesor di perguruan tingg...

Questa voce sull'argomento stagioni delle società calcistiche italiane è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Voce principale: Omegna Calcio 1906. Omegna SportivaStagione 1938-1939Sport calcio Squadra Cusiana Allenatore Cesare Cassanelli Presidente Pasquale Mauro Serie C9º posto nel girone C. 1937-1938 1939-1940 Si invita a seguire il modello di voce Questa voce raccoglie le informazion...

SD Negeri 2 Unggulan MarosInformasiJenisNegeriNomor Pokok Sekolah Nasional40300470Jumlah kelasKelas I sampai kelas VIAlamatLokasiJl. Jenderal Ahmad Yani No. 3 Kelurahan Turikale, Kecamatan Turikale, Kabupaten Maros, Sulawesi Selatan, IndonesiaMoto Bangunan SDN 2 Unggulan Maros di Jl. Jenderal Ahmad Yani No. 3. SD Negeri 2 Unggulan Maros merupakan salah satu sekolah dasar negeri yang terletak di Jl. Jenderal Ahmad Yani No. 3 [1] Kelurahan Turikale, Kecamatan Turikale, Kabupaten Ma...

Ocho Ríos Ciudad Vista de Ocho Ríos Glenn Standish Ocho RíosLocalización de Ocho Ríos en JamaicaCoordenadas 18°23′58″N 77°06′11″O / 18.399444, -77.103056Entidad Ciudad • País JamaicaPoblación (2011) • Total 16 671 hab.[editar datos en Wikidata] Vista de la ciudad Ocho Ríos es una ciudad situada en la costa norte de Jamaica, en la parroquia de Saint Ann. Según el censo de 2011, tiene una población de 16 671...

American baseball player (1963-2004) Baseball player Ken CaminitiThird basemanBorn: (1963-04-21)April 21, 1963Hanford, California, U.S.Died: October 10, 2004(2004-10-10) (aged 41)The Bronx, New York, U.S.Batted: SwitchThrew: RightMLB debutJuly 16, 1987, for the Houston AstrosLast MLB appearanceOctober 7, 2001, for the Atlanta BravesMLB statisticsBatting average.272Home runs239Runs batted in983 Teams Houston Astros (1987–1994) San Diego Padres (1995–1998)...
2019 iK9 Service Dog 200 Race details[1][2][3] Race 4 of 33 in the 2019 NASCAR Xfinity Series season Date March 9, 2019 (2019-03-09)Location ISM Raceway in Avondale, ArizonaCourse Permanent racing facility1 mi (1.6 km)Distance 200 laps, 200 mi (320 km)Pole positionDriver Christopher Bell Joe Gibbs RacingTime 26.871Most laps ledDriver Kyle Busch Joe Gibbs RacingLaps 116WinnerNo. 18 Kyle Busch Joe Gibbs RacingTelevision in the United StatesNetwork FS1Radio...

Buguggiate komune di Italia Tempat Negara berdaulatItaliaDaerah di ItaliaLombardyProvinsi di ItaliaProvinsi Varese NegaraItalia Ibu kotaBuguggiate PendudukTotal3.084 (2023 )GeografiLuas wilayah2,5 km² [convert: unit tak dikenal]Ketinggian306 m Berbatasan denganAzzate Brunello Gazzada Schianno Varese SejarahSanto pelindungVictor Maurus Informasi tambahanKode pos21020 Zona waktuUTC+1 UTC+2 Kode telepon0332 ID ISTAT012025 Kode kadaster ItaliaB258 Lain-lainSitus webLaman resmi Buguggi...

Yvonne PrintempsYvonne Printemps en 1943 (Studio Harcourt).BiographieNaissance 25 juillet 1894ErmontDécès 18 janvier 1977 (à 82 ans)Neuilly-sur-SeineSépulture Cimetière ancien de Neuilly-sur-SeineNom de naissance Yvonne WigniolleNationalité FrançaiseActivité Chanteuse, danseuse, artiste lyrique, actrice, metteure en scèneConjoint Sacha Guitry (de 1919 à 1934) Pierre Fresnay (de 1932 à 1975)Autres informationsTessiture SopranoDistinction Chevalier de la Légion d'honneur (19...

Danau SiombakDanau siombak di sore hariLetakMedanArea permukaan40 hektarKedalaman rata-rata15 m (49 ft)Lihat peta yang diperkecilLihat peta yang diperbesar Danau Siombak adalah sebuah danau buatan dengan luas sekitar 40 hektare, Diameter sekitar 1000 meter,dan kedalaman kurang lebih 12 meter. Danau ini terletak di Kelurahan Paya Pasir, Medan Marelan, Medan, Sumatera Utara. Danau Siombak terletak di antara dua sungai, yaitu Sungai Deli dan Sungai Terjun. Danau ini merupakan bekas gal...

Идеальная причёска навсегдаангл. Perfect Hair Forever Жанры комедия, пародия Создатели Мэтт Майелларо Мэтт Харриган Майк Лаззо[1] Режиссёры Dave Hughes[вд]Мэтт Хэрригэн[вд] Сценаристы Майк Лаццо[вд]Мэтт Хэрригэн[вд]Мэтт Майелларо Роли озвучивали Мэтт Майелларо, Dave Hughes[вд], ...

2016 U.S. presidential election Timeline General election debates Parties Polling national statewide by demographics international Newspaper endorsements primary general Russian interference Russia investigation origins counter-narrative Media coverage Social media International reactions Electors Recounts Faithless electors Vote count Republican Party Primaries Candidates Debates and forums Polling national statewide straw polls Endorsements Results Nominee VP candidate selection Convention...

301st Tactical Fighter Squadron第301飛行隊ActiveOctober 16, 1973 – presentCountry JapanBranch Japan Air Self-Defense ForcePart of3rd Air WingGarrison/HQMisawa Air BaseAircraft flownFighterMitsubishi F-35A Lightning IITrainerKawasaki T-4Military unit The 301st Tactical Fighter Squadron (第301飛行隊 (dai-sann-byaku-ichi-hikoutai)) is a squadron of the 3rd Air Wing of the Japan Air Self-Defense Force based at Misawa Air Base in Misawa, Japan. It is equipped with Mitsubis...

Vaiano ValleStato Italia Regione Lombardia Provincia Milano Città Milano CircoscrizioneMunicipio 5 Altitudine107 m s.l.m. Abitanti153 ab. (Censimento 2011) Nome abitantivaianini Vaiano ValleVaiano Valle (Milano) Vaiano Valle (Vaian in dialetto milanese, AFI: [vaˈjɑ̃:]) è una località rurale, posta nella periferia meridionale di Milano, appartenente al Municipio 5. Indice 1 Storia 2 Note 3 Voci correlate 4 Collegamenti esterni Storia Vajano fu nominata per la prima vo...

MVP情人My MVP Valentine类型偶像劇编剧葉鳳英、楊肅惠、羅彩娟导演劉俊傑、陳銘章主演孫協志、張韶涵、顏行書、陳宇凡制作国家/地区 中華民國(臺灣)标语一段愛與夢想的故事集数18集每集长度90分鐘(含廣告)配乐席裕龍片头曲5566《無所謂》片尾曲5566《我難過》制作制作人陳玉珊、劉俊傑监制陳玉珊剪辑小顧拍攝地點 臺灣摄影蕭聖明制作公司三立電視節目部華視首...

Karl Felix Halm Karl Felix Halm (Monaco di Baviera, 5 aprile 1809 – Monaco di Baviera, 5 ottobre 1882) è stato un filologo classico e bibliotecario tedesco. Indice 1 Biografia 2 Opere 3 Bibliografia 4 Altri progetti 5 Collegamenti esterni Biografia Nel 1849, divenne rettore del Ginnasio Maximilian di Monaco e nel 1856 fu direttore della biblioteca reale e professore dell'Università di Monaco. Opere Halm scrisse delle opere su Cicerone e di altri autori di prosa latina, anche se durante la...