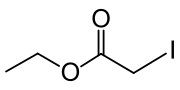
Ethyl iodoacetate
|
|
| Names
|
| Preferred IUPAC name
|
| Other names
Ethyl 2-iodoacetate
|
| Identifiers
|
|
|
|
|
|
|
| ChemSpider
|
|
| ECHA InfoCard
|
100.009.816 
|
|
|
|
| UNII
|
|
|
|
|
InChI=1S/C4H7IO2/c1-2-7-4(6)3-5/h2-3H2,1H3  Y YKey: MFFXVVHUKRKXCI-UHFFFAOYSA-N  Y YInChI=1/C4H7IO2/c1-2-7-4(6)3-5/h2-3H2,1H3 Key: MFFXVVHUKRKXCI-UHFFFAOYAB
|
|
|
| Properties
|
|
|
ICH2CO2CH2CH3
|
| Molar mass
|
214.002 g·mol−1
|
| Appearance
|
Clear, light yellow to orange liquid
|
| Density
|
1.808 g/mL
|
| Boiling point
|
179 to 180 °C (354 to 356 °F; 452 to 453 K)
|
|
|
−97.6·10−6 cm3/mol
|
| Hazards
|
| GHS labelling:[1]
|
|
|
 
|
|
|
Danger
|
|
|
H300, H314
|
|
|
P280, P301+P310+P330, P301+P330+P331, P303+P361+P353, P305+P351+P338+P310
|
| Related compounds
|
|
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
Chemical compound
Ethyl iodoacetate is an organic compound with the chemical formula ICH2CO2CH2CH3. It is a derivative of ethyl acetate.[2][3] Under normal conditions, the compound is a clear, light yellow to orange liquid.
Applications
Used by the British during World War I, it was codenamed SK gas, for the initials of South Kensington, where it was developed.[4]
Like many alkyl iodides, ethyl iodoacetate is an alkylating agent, which makes it useful in organic synthesis, yet toxic. Ethyl iodoacetate is also a lachrymatory agent.
References