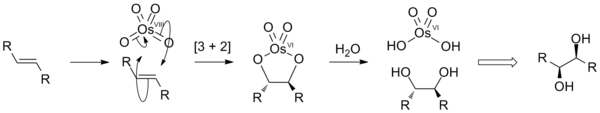Osmium tetroxide
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Maret 2016. Breinigerberg Breinigerberg adalah kampung di Jerman. Jumlah penduduknya adalah 971 (hingga 31 Desember 2005). Cagar alam Cagar alam Schlangenberg Cagar alam Naturpark Hohes Venn - Eifel Pranala luar (Jerman) Breinigerberg Diarsipkan 2020-06-22 di Waybac...

Artikel ini memiliki beberapa masalah. Tolong bantu memperbaikinya atau diskusikan masalah-masalah ini di halaman pembicaraannya. (Pelajari bagaimana dan kapan saat yang tepat untuk menghapus templat pesan ini) Kontributor utama artikel ini tampaknya memiliki hubungan dekat dengan subjek. Artikel ini mungkin memerlukan perapian untuk mematuhi kebijakan konten Wikipedia, terutama dalam hal sudut pandang netral. Silakan dibahas lebih lanjut di halaman pembicaraan artikel ini. (November 2023) (P...

Hong Kong actress (born 1946) Josephine SiaoChinese: 蕭芳芳BornSiao Loeng (蕭亮) (1947-03-13) 13 March 1947 (age 77)Shanghai, ChinaNationalityAustralianAlma materSeton Hall University, Regis UniversityOccupation(s)Actress, television personalityYears active1954–1997Spouses Charlie Chin (m. 1976; div. 1978) Clarence Chang (m. 1980) Children2ParentsXiao Naizhen (father)Cheng Fenghui (mot...

Kevin ArdilovaLahirKevin Ichwal Ardilova19 April 1999 (umur 24)Jakarta, IndonesiaKebangsaanIndonesiaPekerjaanPemeranmodelTahun aktif2014—sekarangTanda tangan Kevin Ichwal Ardilova (lahir 19 April 1999) adalah pemeran dan model berkebangsaan Indonesia. Kevin dinominasikan untuk satu Piala Citra untuk Pemeran Utama Pria Terbaik pada Festival Film Indonesia 2022 melalui penampilannya dalam film Autobiography.[1] Karier Pada 2017, ia memulai debutnya di dunia perfilman dengan...

Cet article est une ébauche concernant les forces armées des États-Unis et la science. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Defense Advanced Research Projects AgencyHistoireFondation 1958CadreSigle (en) DARPAType Agence fédérale des États-Unis (département de la Défense des États-Unis)Domaines d'activité Recherche et développement, technologie militaire, Military researchSiège Arlingt...

Italian engraver and teacher Giacomo Francia, Brawl of Bruttobuono, Rhode Island School of Design Museum, 1601 Francesco Villamena (1564–1624) was an Italian engraver, drawing teacher and art collector.[1] Villamena was born in Assisi. He studied under Cornelis Cort. Others state he was a follower of Agostino Carracci. Villamena produced primarily works of religious and historical subjects. He died in Rome in 1624.[1] References ^ a b British Museum: Francesco Villamena Furt...

Voce principale: Law & Order: UK. La sesta stagione della serie televisiva Law & Order: UK è stata trasmessa per la prima volta sul canale statunitense BBC America dal 28 settembre al 9 novembre 2011 e trasmessa solo successivamente dal canale inglese ITV dal 6 gennaio al 17 febbraio 2012; questo è accaduto perché, nei mercati internazionali, la serie viene venduta in stagioni produttive di 13 episodi, mentre nel Regno Unito queste vengono spezzate in due blocchi che poi vengono t...

Державний комітет телебачення і радіомовлення України (Держкомтелерадіо) Приміщення комітетуЗагальна інформаціяКраїна УкраїнаДата створення 2003Керівне відомство Кабінет Міністрів УкраїниРічний бюджет 1 964 898 500 ₴[1]Голова Олег НаливайкоПідвідомчі ор...

Équilly L’église Sainte-Anne. Administration Pays France Région Normandie Département Manche Arrondissement Avranches Intercommunalité Communauté de communes de Granville, Terre et Mer Maire Mandat Claire Rousseau 2020-2026 Code postal 50320 Code commune 50174 Démographie Gentilé Équillais Populationmunicipale 194 hab. (2021 ) Densité 34 hab./km2 Géographie Coordonnées 48° 50′ 27″ nord, 1° 23′ 09″ ouest Altitude 121 mMin. 5...

SMA Negeri 13 BekasiInformasiDidirikan5 Juni 1945 (sebagai USB SMA Negeri 13 Bekasi)8 Oktober 2007 (sebagai SMA Negeri 13 Bekasi)JenisNegeriAkreditasiA (Sangat Baik)[1][2]Nomor Statistik Sekolah301026510013[1]Nomor Pokok Sekolah Nasional20231719[2]Kepala SekolahHasim, S.Pd., M.M., CHMP., CNSHP., CNICP., CNHRP., CNBLP.[3]Jumlah kelasX (11 kelas) XI (10 kelas) XII (10 kelas)Jurusan atau peminatanMIPA dan IPSRentang kelasX MIPA, X IPS, XI MIPA, X...

Führer dan Kanselir Reich Rakyat JermanFührer und Reichskanzler des deutschen VolkesBekas jabatan politikBendera HitlerAdolf HitlerPendahuluPaul von Hindenburg(sebagai presiden)[1]Dirinya sendiri(sebagai kanselir)PenggantiKarl Dönitz (sebagai presiden)Joseph Goebbels(sebagai kanselir)Pejabat pertamaAdolf HitlerPejabat terakhirAdolf HitlerGayaYang Mulia/ Mëin FührerKediaman resmiReich ChancelleryPelantikReichstag, Pakta Pengaktifan 1933Jabatan dimulai2 Agustus 1934Jabatan berakhir...

Town in Kent, England This article is about the town in Kent, England. For other uses, see Gravesend (disambiguation). Town in EnglandGravesendTownNew Road, Gravesend in 2009Kent coat of armsGravesendLocation within KentPopulation58,102 [1]OS grid referenceTQ647740DistrictGraveshamShire countyKentRegionSouth EastCountryEnglandSovereign stateUnited KingdomPost townGRAVESENDPostcode districtDA11, DA12Dialling code01474PoliceKentFireKentAmbulanceSouth E...

Medali Silver Star Silver Star Medal, yang secara tak resmi disebut Silver Star, adalah penghargaan pribadi tertinggi ketiga dalam militer Amerika Serikat untuk pejuang dalam penyerangan. Silver Star Medal biasanya dianugerahi kepada para anggota Angkatan Bersenjata Amerika Serikat atas jasa-jasa dalam tindakan melawan musuh Amerika Serikat. Pranala luar Wikimedia Commons memiliki media mengenai Silver Star. Silver Star database at MilitaryTimes.com Awards and Decorations Air Force Personnel ...

Virginia House of Delegates election, 1985 ← 1983 November 5, 1985 1987 → All 100 seats in the Virginia House of Delegates51 seats needed for a majorityTurnout53.0%[1] Majority party Minority party Leader A. L. Philpott Vince Callahan Party Democratic Republican Leader since January 9, 1980 January 13, 1982 Leader's seat 11th 34th Last election 65 34 Seats won 65 33 Seat change 1 Popular vote 630,701 427,656 Percentage...

ماري ساكس التينبورغ (بالألمانية: Marie von Sachsen-Altenburg) معلومات شخصية اسم الولادة (بالألمانية: Alexandrine Marie Wilhelmine Katharine Charlotte Theresia Henriette Luise Pauline Elisabeth Friederike Georgine von Sachsen-Altenburg) الميلاد 14 أبريل 1818(1818-04-14)هيلدبورغهاوزن الوفاة 9 يناير 1907 (88 سنة) غموندن مكان الدفن غمون�...

English canal linking the south Cheshire town of Nantwich with the River Dee at Chester Chester CanalChester Canal basin, on the Wirral Line of the Ellesmere Canal, at Raymond Street, near the junction with the Chester Canal and the River DeeSpecificationsMaximum boat length72 ft 0 in (21.95 m)(originally 80 ft 0 in or 24.38 m)Maximum boat beam9 ft 0 in (2.74 m)(originally 14 ft 9 in or 4.50 m)Locks14StatusNavigableNavigation authori...

Japanese manga series Papa and Daddy's Home CookingFirst tankōbon volume coverパパと親父のウチご飯(Papa to Oyaji no Uchi Gohan)GenreCooking[1] MangaWritten byYuu ToyotaPublished byShinchoshaEnglish publisherNA: MedibangMagazineMonthly Comic @BunchDemographicSeinenOriginal runApril 21, 2014 – July 21, 2020Volumes13 (List of volumes) MangaPapa to Oyaji no Uchi NomiWritten byYuu ToyotaPublished byShinchoshaMagazineGo Go BunchComic Bunch WebDemographicSein...

Deputy head of government in the USSR First Deputy Premier of theSoviet UnionПервый заместитель Председателя Совета Народных Комиссаров СССР (1923–1946)Первый заместитель Председателя Совета министров СССР (1946–1991)Первый заместитель премьер-министра СССР (1991)Coat of arms of the Soviet UnionLongest servingKirill Mazurov26 March 1965 – 28 Novemb...

Serie B1 2021-2022 Competizione Serie B1 Sport Pallavolo Edizione 32ª Organizzatore FIPAV Date dal 16 ottobre 2022al 4 giugno 2022 Luogo Italia Partecipanti 72 Risultati Promozioni Esperia Lecco Picco Montale Offanengo Perugia Sant'Anna Retrocessioni Vedi elenco Statistiche Incontri disputati 818 Cronologia della competizione 2020-2021 2022-2023 Manuale La Serie B1 2021-2022 si è svolta dal 16 ottobre 2021 al 4 giugno 2022: al torneo hanno ...

Proposed political union of New Brunswick, Nova Scotia and Prince Edward Island For maritime industry labour unions, see Trade union. The three Maritime provinces. Maritime Union (French: Union des Maritimes) is a proposed political union of the three Maritime provinces of Canada – New Brunswick, Nova Scotia, and Prince Edward Island – to form a single new province.[1] The idea has been proposed at various times throughout Canadian history. Most recently, it was reintroduced in No...






![{\displaystyle {\ce {Os + 2O2 ->[\Delta T] OsO4}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8d2c2ac06e64cd164869ed5506f13c3cfa320d70)