Dichlorine monoxide
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Akitsu Maru pada tahun 1944 Sejarah Kekaisaran Jepang Nama Akitsu MaruPembangun Harima, Harima[1]Pasang lunas 17 September 1940Diluncurkan 24 September 1941Selesai 30 Januari 1942Nasib Tenggelam pada 15 November 1944 Ciri-ciri umum Jenis Kapal serbu amfibiBerat benaman 11.800 ton panjang (11.989 t) (standar)[1]Panjang 471 ft 7 in (143,74 m) (perpendikuler)[1]Lebar 64 ft (20 m)[1]Sarat air 25 ft 9 in (7,85 m) (maksim...

You can help expand this article with text translated from the corresponding article in Spanish. (May 2010) Click [show] for important translation instructions. View a machine-translated version of the Spanish article. Machine translation, like DeepL or Google Translate, is a useful starting point for translations, but translators must revise errors as necessary and confirm that the translation is accurate, rather than simply copy-pasting machine-translated text into the English Wikipedi...

Ivan Kolev Informasi pribadiNama lengkap Ivan Venkov KolevTanggal lahir 14 Juli 1957 (umur 66)Tempat lahir Sofia, BulgariaKarier senior*Tahun Tim Tampil (Gol) Lokomotiv Sofia Sliven FC Akademik VIF FC CSKA Sofia Kepelatihan1982–1987 Levski1987–1992 Iskar1993–1994 Slavia1995–-1996 Punav Ruse1996-1997 Kremnikevci1997-1998 Bulgaria U-19 (asisten)1998 Bulgaria U-191999 Persija2000-2002 Bulgaria U-202002-2004 Indonesia2004-2005 Myanmar2006 Mitra Kukar2007 Persipura2007 Indonesia2008�...

Nama-nama yang disebutkan disini adalah nama tokoh-tokoh yang tergabung dalam Kepanitiaan Badan Penyelidik Usaha-usaha Persiapan Kemerdekaan Indonesia dan Panitia Persiapan Kemerdekaan Indonesia Dokuritsu Junbi Chosakai Dokuritsu Junbi Cosakai atau yang sering dikenal dengan Badan Penyelidik Usaha-usaha Persiapan Kemerdekaan Indonesia (BPUPKI) adalah sebuah Badan yang dibentuk oleh Pemerintah Angkatan Darat XVI Jepang yang berkedudukan di Jakarta (selengkapnya baca artikel BPUPKI) ini berangg...
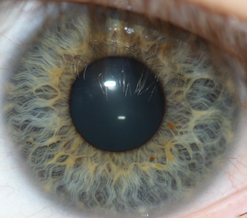
Method of biometric identification Iris recognition biometric systems apply mathematical pattern-recognition techniques to images of the irises of an individual's eyes. Iris recognition is an automated method of biometric identification that uses mathematical pattern-recognition techniques on video images of one or both of the irises of an individual's eyes, whose complex patterns are unique, stable, and can be seen from some distance. The discriminating powers of all biometric technologies d...

Commune in Île-de-France, FranceCourbevoieCommuneCourbevoie Municipal Library Coat of armsParis and inner ring départementsLocation of Courbevoie CourbevoieShow map of FranceCourbevoieShow map of Île-de-France (region)Coordinates: 48°53′52″N 2°15′11″E / 48.8978°N 2.2531°E / 48.8978; 2.2531CountryFranceRegionÎle-de-FranceDepartmentHauts-de-SeineArrondissementNanterreCantonCourbevoie-1 and 2IntercommunalityGrand ParisGovernment • Mayor (2020&#...

Miguel Ángel de Quevedo UbicaciónCoordenadas 19°20′47″N 99°10′52″O / 19.346395, -99.18103Dirección Av. Universidad y Miguel Ángel de QuevedoCol. Chimalistac y Romero de Terreros.Localidad Álvaro Obregón y Coyoacán, Ciudad de México Datos de la estaciónPunto kilométrico 18.4 kmInauguración 30 de agosto de 1983 (40 años)Pasajeros Pasajeros en 2023 11,335,490[1] (12.64%) Ranking en 2023 24°/195 ( 4°/20)Conexiones Línea 7 del Servicio de Transp...

MuftiAbdur Rahmanআব্দুর রহমান চাটগামীPersonalBornAbdur Rahman1920Imam Nagar, Fatikchhari, Chittagong District, Bengal Presidency, British IndiaDied10 November 2015(2015-11-10) (aged 94–95)Dhaka, BangladeshReligionIslamNationalityBangladeshiRegionBangladeshDenominationSunniJurisprudenceHanafiMovementDeobandiMain interest(s)Islamic economics, Hadith, Islamic jurisprudenceMuslim leaderTeacherSultan Ahmad Nanupuri Influenced by Azizul Haq Abdur Rah...

County-class heavy cruiser built in the late 1920s For other ships with the same name, see HMS Kent. History United Kingdom NameKent NamesakeKent BuilderChatham Dockyard Laid down15 November 1924 Launched16 March 1926 Commissioned25 June 1928 IdentificationPennant number: 54 FateSold for scrap, 22 January 1948 General characteristics (as built) Class and typeCounty-class heavy cruiser Displacement 9,850 long tons (10,010 t) (standard load) 13,520 long tons (13,740 t) (deep load) Len...

لمعانٍ أخرى، طالع سلطان أباد (توضيح). سلطان أباد تقسيم إداري البلد إيران إحداثيات 37°50′39″N 45°01′42″E / 37.84416667°N 45.02833333°E / 37.84416667; 45.02833333 تعديل مصدري - تعديل سلطان أباد هي قرية في مقاطعة أرومية، إيران. عدد سكان هذه القرية هو 567 في سنة 2006.[1] مراجع ...

American baseball player (born 1978) Baseball player Chris BootcheckBootcheck with the Yokohama BayStars in 2010PitcherBorn: (1978-10-24) October 24, 1978 (age 45)La Porte, Indiana, U.S.Batted: RightThrew: RightProfessional debutMLB: September 9, 2003, for the Anaheim AngelsNPB: May 5, 2010, for the Yokohama BayStarsKBO: July 15, 2011, for the Lotte GiantsLast appearanceNPB: July 16, 2010, for the Yokohama BayStarsKBO: October 5...

British professional wrestler (1946–1998) For the American wrestler, see Haystacks Calhoun. Giant HaystacksRuane in 1981Birth nameMartin Austin RuaneBorn(1946-10-10)10 October 1946Camberwell, London, EnglandDied29 November 1998(1998-11-29) (aged 52)Prestwich, Greater Manchester, EnglandCause of deathLymphomaSpouse(s)Rita BoylanChildren3Professional wrestling careerRing name(s)Giant HaystacksHaystacks CalhounLoch NessLoch Ness MonsterLuke McMastersBilled height6 ft 11 in (211&...

Town in West Coast, New Zealand Town in West Coast, New ZealandHokitikaTownHokitika townshipCoordinates: 42°42′56″S 170°58′5″E / 42.71556°S 170.96806°E / -42.71556; 170.96806CountryNew ZealandRegionWest CoastDistrictWestland DistrictWardHokitikaSettled by Europeans1864ElectoratesWest Coast-TasmanTe Tai TongaGovernment • Territorial authorityWestland District Council • Regional councilWest Coast Regional Council • Mayor of...

يفتقر محتوى هذه المقالة إلى الاستشهاد بمصادر. فضلاً، ساهم في تطوير هذه المقالة من خلال إضافة مصادر موثوق بها. أي معلومات غير موثقة يمكن التشكيك بها وإزالتها. (يوليو 2019) هذه المقالة تحتاج للمزيد من الوصلات للمقالات الأخرى للمساعدة في ترابط مقالات الموسوعة. فضلًا ساعد في تحسي...

WellsvilleThe Wellsville depot in May 2016.General informationLocation10 Depot Street, Wellsville, New YorkLine(s)Main Line (Allegany Division)Platforms1 side platformTracks2ConstructionPlatform levels1Other informationStation code4413[1]HistoryOpenedMay 14, 1851; 173 years ago (May 14, 1851)[2]ClosedJanuary 6, 1970; 54 years ago (January 6, 1970)[3]Rebuilt1911; 113 years ago (1911)Former services Preceding station Erie ...

Questa voce sull'argomento calciatori argentini è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Fernando MonettiNazionalità Argentina Altezza184 cm Peso77 kg Calcio RuoloPortiere Squadra Sarmiento (J) CarrieraGiovanili Gimnasia (LP) Squadre di club1 2010-2014 Gimnasia (LP)146 (-126)2015-2018 Lanús57 (-51)2018 Atlético Nacional13 (-6)2019→ San Lorenzo7 (-9)2020-...
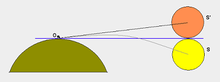
提示:此条目页的主题不是大氣繞射。 顯示太陽在日出和日落時位移的圖。 大氣折射(又稱:蒙氣差(蒙氣即行星的大氣)、折光差)即原本直線前進的光或其它電磁波在穿越大氣層時,因為空氣密度隨著高度變化所產生的偏折。這種折射是光通過空氣時因為密度的增加使速度降低(折射率增加)。大氣折射在近地面時會產生海市蜃樓,讓遠方的物體出現或蕩漾,...

Pro-soviet revolution in Tashkent This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (December 2022) (Learn how and when to remove this message) Tashkent RebellionPart of Russian Civil WarMap of Tashkent and surrounding area, created c. 1916DateSeptember 1917 – 13 November 1917 [O.S. September 1917 – 31 October 1917]LocationTashkent, Russian Empire (n...

A 2007 map of Burkina Faso, including main and secondary roads, major airports, and railroad lines. Transport in Burkina Faso consists primarily of road, air and rail transportation. The World Bank classified country's transportation as underdeveloped but noted that Burkina Faso is a natural geographic transportation hub for West Africa.[1] Highways S. T. M. B. (Service de Transport Mixte Bangrin.) A market during a break in the bus journey from Ouagadougou to Bobo-Dioulasso. Boromo,...

Volpedo komune di Italia Tempat Negara berdaulatItaliaDaerah di ItaliaPiemonteProvinsi di ItaliaProvinsi Alessandria NegaraItalia Ibu kotaVolpedo PendudukTotal1.150 (2023 )GeografiLuas wilayah10,48 km² [convert: unit tak dikenal]Ketinggian182 m Berbatasan denganCasalnoceto Godiasco Monleale Montemarzino Pozzol Groppo Volpeglino SejarahHari liburpatronal festival (en) Organisasi politikAnggota dariThe most beautiful villages in Italy (en) Informasi tambahanKode pos15059 Zona waktuU...







