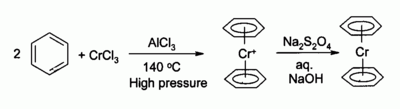
Chromium(III) chloride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Untuk orang lain dengan nama yang sama, lihat Richard Stanton. Richard StantonRichard Stanton pada 1919Lahir(1876-10-08)8 Oktober 1876Iowa, Amerika SerikatMeninggal22 Mei 1956(1956-05-22) (umur 79)Los Angeles, California, Amerika SerikatPekerjaanPemeran, sutradaraTahun aktif1911–1925 Richard Stanton (8 Oktober 1876 – 22 Mei 1956) adalah seorang pemeran dan sutradara Amerika Serikat pada era film bisu. Ia tampil dalam 68 film antara 1911 dan 1916. Ia juga menyutrada...

Country in the Caribbean This article is about the country. For the islands of the sovereign state, see Trinidad and Tobago. For other uses, see Trinidad (disambiguation) and Tobago (disambiguation). Republic of Trinidad and Tobago Flag Coat of arms Motto: Together we aspire, Together we achieveAnthem: Forged from the Love of LibertyCapitalPort of Spain10°40′0″N 61°30′27″W / 10.66667°N 61.50750°W / 10.66667; -61.50750Largest cityChaguanas 10°31�...

Indian politician Hukumdev Narayan YadavYadav receiving the Outstanding Parliamentarian Award in 2018.Member of Parliament, Lok SabhaIn office2009-2019;1999-2004Preceded byShakeel Ahmad, INC(1998-1999 & 2004-2009)Succeeded byAshok Kumar Yadav, BJPConstituencyMadhubani Personal detailsBorn (1939-11-17) 17 November 1939 (age 84)Bijuli, DarbhangaCitizenshipIndiaNationalityIndianPolitical partyBharatiya Janata PartySpouseSudesh Yadav[1]Children3 including Ashok Kumar YadavResiden...

Public library system This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Mid-Continent Public Library – news · newspapers · books · scholar · JSTOR (June 2023) (Learn how and when to remove this template message) Mid-Continent Public Library39°06′42″N 94°23′30″W / 39.11164°N 94.39154�...

Austrian tennis player Alexander ErlerErler at the 2022 Internationaux de Tennis de BloisCountry (sports) AustriaResidenceKufstein, AustriaBorn (1997-10-27) 27 October 1997 (age 26)Innsbruck, AustriaHeight1.93 m (6 ft 4 in)Turned pro2015PlaysRight-handed (two-handed backhand)CoachDaniel Huber, Thomas WeindorferPrize money$657,617SinglesCareer record1–1 (in ATP Tour events)Career titles0Highest rankingNo. 322 (4 October 2021)Current rank...

Lovisa Grant Nazionalità Svezia Sci alpino Specialità Slalom gigante, slalom speciale, combinata Squadra IFK Lidingö SLK Termine carriera 2017 Palmarès Competizione Ori Argenti Bronzi Mondiali juniores 0 1 0 Per maggiori dettagli vedi qui Modifica dati su Wikidata · Manuale Lovisa Grant (6 marzo 1995) è un'ex sciatrice alpina svedese. Indice 1 Biografia 2 Palmarès 2.1 Mondiali juniores 2.2 Coppa Europa 2.3 Campionati svedesi 3 Collegamenti esterni Biografia Attiva in...

High Priest during the Second Temple period Onias III from Hartmann Schedel's Nuremberg Chronicles (1493) Onias III (Hebrew: חוֹנִיּוֹ Ḥōnīyyō), son of Simon II, was Jewish High Priest during the Second Temple period. He is described in scriptures as a pious man who opposed the Hellenization of Judea.[1] He was succeeded by his brother Jason in 175 BCE. Politics of the office The Seleucid Empire controlled Jerusalem during Onias' tenure and Seleucus IV Philopator (reigne...

Este artículo o sección necesita ser wikificado, por favor, edítalo para que cumpla con las convenciones de estilo.Este aviso fue puesto el 27 de mayo de 2021. Este artículo o sección necesita referencias que aparezcan en una publicación acreditada. Busca fuentes: «Looney Tunes» – noticias · libros · académico · imágenesEste aviso fue puesto el 31 de octubre de 2013. No debe confundirse con Luny Tunes. Para ver a su serie hermana, véase Merrie Melodies. Loo...

Sceaux 行政国 フランス地域圏 (Région) イル=ド=フランス地域圏県 (département) オー=ド=セーヌ県郡 (arrondissement) アントニー郡小郡 (canton) 小郡庁所在地INSEEコード 92071郵便番号 92330市長(任期) フィリップ・ローラン(2008年-2014年)自治体間連合 (fr) メトロポール・デュ・グラン・パリ人口動態人口 19,679人(2007年)人口密度 5466人/km2住民の呼称 Scéens地理座標 北緯48度4...

Matt LeBlanc nel 2013 Matt LeBlanc, all'anagrafe Matthew Steven LeBlanc (Newton, 25 luglio 1967[1]), è un attore statunitense. Ha ricevuto riconoscimenti nazionali ed internazionali per la sua interpretazione di Joey Tribbiani nella popolare sitcom Friends della NBC, trasmessa dal 1994 al 2004; per il suo lavoro in Friends, LeBlanc ha ricevuto tre nomination agli Emmy Award. Ha anche interpretato una versione fittizia di se stesso nella serie comica della BBC / Showtime Episodes (201...
周處除三害The Pig, The Snake and The Pigeon正式版海報基本资料导演黃精甫监制李烈黃江豐動作指導洪昰顥编剧黃精甫主演阮經天袁富華陳以文王淨李李仁謝瓊煖配乐盧律銘林孝親林思妤保卜摄影王金城剪辑黃精甫林雍益制片商一種態度電影股份有限公司片长134分鐘产地 臺灣语言國語粵語台語上映及发行上映日期 2023年10月6日 (2023-10-06)(台灣) 2023年11月2日 (2023-11-02)(香�...

土库曼斯坦总统土库曼斯坦国徽土库曼斯坦总统旗現任谢尔达尔·别尔德穆哈梅多夫自2022年3月19日官邸阿什哈巴德总统府(Oguzkhan Presidential Palace)機關所在地阿什哈巴德任命者直接选举任期7年,可连选连任首任萨帕尔穆拉特·尼亚佐夫设立1991年10月27日 土库曼斯坦土库曼斯坦政府与政治 国家政府 土库曼斯坦宪法 国旗 国徽 国歌 立法機關(英语:National Council of Turkmenistan) ...

此條目需要补充更多来源。 (2021年7月4日)请协助補充多方面可靠来源以改善这篇条目,无法查证的内容可能會因為异议提出而被移除。致使用者:请搜索一下条目的标题(来源搜索:美国众议院 — 网页、新闻、书籍、学术、图像),以检查网络上是否存在该主题的更多可靠来源(判定指引)。 美國眾議院 United States House of Representatives第118届美国国会众议院徽章 众议院旗...

بَحْرُ المضارع الاكتشاف الواضع الخليل بن أحمد الفراهيدي الخصائص الوزن مفاعيل فاعلاتن ••• مفاعيل فاعلاتن المفتاح تعد المضارعات ••• مفاعيل فاعلاتن بوابة الأدب العربي بوابة الشعر تعديل مصدري - تعديل المضارع أهل العروض بحر شعر من البحور المشتركة بين العرب�...

This article relies excessively on references to primary sources. Please improve this article by adding secondary or tertiary sources. Find sources: United States v. Knotts – news · newspapers · books · scholar · JSTOR (November 2019) (Learn how and when to remove this message) 1983 United States Supreme Court caseUnited States v. KnottsSupreme Court of the United StatesArgued December 6, 1982Decided March 2, 1983Full case nameUnited States v. KnottsC...

معركة الزميل جزء من الفتح الإسلامي لفارسحملات خالد بن الوليد موقع الزميل على الخارطة، حيث معارك خالد بن الوليد معلومات عامة التاريخ رمضان 12 هـ الموافق نوفمبر 633م الموقع العراق النتيجة انتصار جيش المسلمين المتحاربون الخلفاء الراشدين الساسانيونالعرب النصارى القادة خالد ب...

Questa voce o sezione sull'argomento nobili tedeschi non cita le fonti necessarie o quelle presenti sono insufficienti. Puoi migliorare questa voce aggiungendo citazioni da fonti attendibili secondo le linee guida sull'uso delle fonti. Leopoldo IIIRitratto del principe Leopoldo III di LippePrincipe di LippeStemma In carica1º gennaio 1851 –8 dicembre 1875 PredecessoreLeopoldo II SuccessoreValdemaro TrattamentoSua altezza serenissima NascitaDetmold, 1º settembre 1821 MorteD...

Place in Lower Carniola, SloveniaZgornje VodaleZgornje VodaleLocation in SloveniaCoordinates: 45°57′35.57″N 15°14′22.46″E / 45.9598806°N 15.2395722°E / 45.9598806; 15.2395722Country SloveniaTraditional regionLower CarniolaStatistical regionLower SavaMunicipalitySevnicaArea • Total1.49 km2 (0.58 sq mi)Elevation494.7 m (1,623.0 ft)Population (2002) • Total37[1] Zgornje Vodale (pronounced [ˈzɡ...

The Ugly TruthPoster BioskopSutradaraRobert LuketicProduserKatherine Heigl Karen McCullah Lutz Kirsten Smith Tom RosenbergGary LucchesiSkenarioNicole EastmanKaren McCullah Lutz Kirsten SmithCeritaNicole EastmanPemeranKatherine Heigl Gerard Butler Bonnie Somerville Yvette Nicole BrownPenata musikAaron ZigmanSinematograferRussell CarpenterPenyuntingLisa Zeno ChurginPerusahaanproduksiRelativity Media Lakeshore EntertainmentDistributorColumbia PicturesTanggal rilis 24 Juli 2009 (2009-0...

Pour les articles homonymes, voir Diagramme pvT. L'équation d'état pvT est la relation qui lie, pour un système : la pression, exprimée en pascals (Pa) ; le volume spécifique v = 1/ρ, exprimé en mètres cubes par kilogramme (m3·kg−1) ; la température thermodynamique T, exprimée en kelvins (K). Elle est utilisée pour représenter la compressibilité des polymères, notamment dans les problèmes d'injection plastique. On trace typiquement un faisceau de courbes v = ...