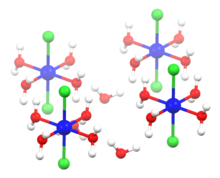
Cobalt(II) chloride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Artikel ini memerlukan pemutakhiran informasi. Harap perbarui artikel dengan menambahkan informasi terbaru yang tersedia. Artikel atau sebagian dari artikel ini mungkin diterjemahkan dari IPhone XR di en.wikipedia.org. Isinya masih belum akurat, karena bagian yang diterjemahkan masih perlu diperhalus dan disempurnakan. Jika Anda menguasai bahasa aslinya, harap pertimbangkan untuk menelusuri referensinya dan menyempurnakan terjemahan ini. Anda juga dapat ikut bergotong royong pada ProyekWiki P...

Harian Metro SiantarTipeSurat kabar harianPemilikJawa Pos GroupPenerbitPT Siantar Media PersDidirikan10 April 2003 (sebagai POS METRO SIANTAR) 2006BahasaIndonesiaPusatJl. Sangnaualuh, Megaland Blok A No.24 Pematangsiantar - Sumatera UtaraSurat kabar saudariNew Tapanuli, Metro Asahan, Metro TabagselSitus webmetrodaily.jawapos.com METRO SIANTAR adalah surat kabar harian yang terbit di Pematangsiantar, Sumatera Utara, Indonesia. Surat kabar ini merupakan usaha Jawa Pos Group. Kantor pusatnya ter...

American politician Frank CaprioCaprio in 200929th General Treasurer of Rhode IslandIn officeJanuary 7, 2007 – January 4, 2011GovernorDon CarcieriPreceded byPaul J. TavaresSucceeded byGina Raimondo Personal detailsBorn (1966-05-10) May 10, 1966 (age 57)Providence, Rhode Island, U.S.Political partyDemocraticChildren2ParentFrank Caprio (father)EducationHarvard University (BA)Suffolk University (JD)OccupationPoliticianProfessionBanker, lawyer Frank T. Caprio (born May 10, 1966) i...

County in Kansas, United States Not to be confused with Pawnee, Kansas. County in KansasPawnee CountyCountyPawnee County Courthouse in Larned (2009)Location within the U.S. state of KansasKansas's location within the U.S.Coordinates: 38°09′N 99°12′W / 38.15°N 99.2°W / 38.15; -99.2Country United StatesState KansasFoundedFebruary 26, 1867Named forPawnee tribeSeatLarnedLargest cityLarnedArea • Total755 sq mi (1,960 km2) �...

Плотность населения Аляски Население Аляски Год Численность Изменение 1880 33 426 - 1890 32 052 -4.1 % 1900 63 592 98,4 % 1910 64 356 1,2 % 1920 55 036 -14,5 % 1930 59 278 7,7 % 1940 72 524 22,3 % 1950 128 643 77,4 % 1960 226 167 75.8 % 1970 300 382 32,8 % 1980 401 851 33,8 % 1990 550 043 36,9 % 2000 626 932 14,0 % 2010 710 231 13,3 % 2020 736 081 3,6 % Аляс�...

Daley Sinkgraven Sinkgraven nel 2016 Nazionalità Paesi Bassi Altezza 180 cm Peso 67 kg Calcio Ruolo Centrocampista Squadra Las Palmas Carriera Giovanili 2006-2013 Heerenveen Squadre di club1 2013-2015 Heerenveen36 (4)2015-2019 Ajax63 (1)2019-2023 Bayer Leverkusen59 (0)2023- Las Palmas6 (0) 1 I due numeri indicano le presenze e le reti segnate, per le sole partite di campionato.Il simbolo → indica un trasferimento in prestito. Statistiche aggiornate al&...

This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article's lead section may be too short to adequately summarize the key points. Please consider expanding the lead to provide an accessible overview of all important aspects of the article. (November 2023) This article needs to be updated. The reason given is: Needs more information on aftermath & legacy. Please help update this art...

2022 award ceremony 19th British Academy Games AwardsDate30 March 2023LocationQueen Elizabeth HallHosted byFrankie WardBest GameVampire SurvivorsMost awardsGod of War Ragnarök (6)Most nominationsGod of War Ragnarök (15) ← 18th · British Academy Games Awards · 20th → The 19th British Academy Video Game Awards were hosted by the British Academy of Film and Television Arts on 30 March 2023 to honour the best video games of 2022. Held at the Queen Elizabeth Hal...

Військово-музичне управління Збройних сил України Тип військове формуванняЗасновано 1992Країна Україна Емблема управління Військово-музичне управління Збройних сил України — структурний підрозділ Генерального штабу Збройних сил України призначений для планува...

This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article relies excessively on references to primary sources. Please improve this article by adding secondary or tertiary sources. Find sources: Maxwell Lord – news · newspapers · books · scholar · JSTOR (October 2021) (Learn how and when to remove this message) This article may be written from a ...

Indian Bengali magazine for young adults This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Unish-Kuri – news · newspapers · books · scholar · JSTOR (June 2024) (Learn how and when to remove this message) Unish-KuriCover of Unish-Kuri, December 2017FrequencyfortnightlyPublisherABP LtdFirst issue19 June 2004Base...

East Forest Park Branch Library East Forest Park is a neighborhood in the south central part of Springfield, Massachusetts. The neighborhood borders East Longmeadow, Forest Park, and the Sixteen Acres neighborhood. It is a primarily residential middle-class neighborhood. Neighborhood East Forest Park contains a wide variety of pre and post-World War II homes on the hillside of Watershops Pond, Springfield's largest body of water (other than the Connecticut River) and custom-built colonials an...

Motor vehicle BMW X5OverviewManufacturerBMWModel codeE53ProductionSeptember 1999 – September 2006 (467,995 Produced)Model years2000-2006AssemblyUnited States: Greer, South Carolina (Plant Spartanburg)Mexico: Toluca (BMW Mexico)DesignerPeter Gabath (1995)Chris Bangle (1996, LCI: 2002)Wolfgang Reitzle (1996)Frank Stephenson (1996)Henrik FiskerBody and chassisClassMid-size luxury SUVLayoutFront-engine, all-wheel-driveRelatedBMW 5 Series (E39)Range Rover (L322)PowertrainEnginePetrol:3.0&#...

Uni Republik Sosialis Soviet Keanggotaan Perserikatan Bangsa-BangsaKeanggotaanBekas anggota penuhTanggal1945 (1945) – 1991 (1991)Kursi DK PBBPermanenDuta Besar Andrei Gromyko 1946–1948 Yakov Malik 1948–1952 Valerian Zorin 1952–1953 Andrey Vyshinsky 1953–1954 Arkady Sobolev 1955–1960 Valerian Zorin 1960–1963 Nikolai Fedorenko 1963–1968 Yakov Malik 1968–1976 Oleg Troyanovsky 1976–1986 Yuri Dubinin 1986 Alexander Belonogov 1986–1990 Yuli Vorontsov 1990–1991 ...

Yugo-ZapadnayaЮго-ЗападнаяStasiun Metro MoskwaPemilikMoskovsky MetropolitenJalur!C 1 Jalur Sokolnicheskaya Jumlah peron1Jumlah jalur2LayananBus: 66, 144, 196, 226, 227, 272, 261, 272, 281, 611, 611с, 630, 642, 688, 699, 707, 707к, 718, 720, 735, 752, 785, 802, 816, 844Trolleybus: 34,62,84KonstruksiJenis strukturShallow column triple-span stationKedalaman8 meter (26 ft)Tinggi peron1ParkirNoInformasi lainKode stasiun019SejarahDibuka30 Desember 1963Operasi...

المحكمة العسكرية العليا الخاصة / محكمة الشعب / محكمة المهداوي أحد جلسات الحكم في محكمة المهداوي رئيس المحكمة العقيد فاضل عباس المهداوي المحكمة العسكرية العليا الخاصة واسمها الرسمي محكمة الشعب او محكمة المهداوي، وهي محكمة أسست عام 1958م، بأمر من الزعيم عبد الكريم قاسم رئيس و...

У Вікіпедії є статті про інших людей із прізвищем Франко. Петро Франко Поручник (обер-лейтенант) Сотник ПолковникПетро Франко у формі поручника УГАЗагальна інформаціяНародження28 червня 1890(1890-06-28)с. Нагуєвичі, Австро-УгорщинаСмертьне раніше 28 червня 1941невідом...

1382 tax revolt in France The Harelle (French pronunciation: [aʁɛl]; from haro) was a revolt that occurred in the French city of Rouen in 1382 and followed by the Maillotins uprising a few days later in Paris, as well as numerous other revolts across France in the subsequent week.[1] France was in the midst of the Hundred Years' War, and had seen decades of warfare, widespread destruction, high taxation, and economic decline, made worse by bouts of plague. In Rouen, the seco...

Graphs are unavailable due to technical issues. There is more info on Phabricator and on MediaWiki.org.Total IPC crimes (only IPC crime rate, not IPC & SLL crimes)[1] States and union territories of India ordered by Area Population GDP (per capita) Abbreviations Access to safe drinking water Availability of toilets Capitals Child nutrition Crime rate Ease of doing business Electricity penetration Exports Fertility rate Forest cover Highest point HDI Home ownership Household size ...
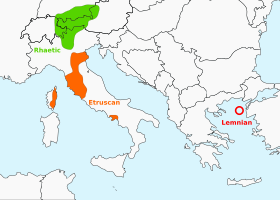
لغات تيرهينية النسب كيان كيانكيان لاماديlanguoid (en) لغةلغة بشريةلغات تيرهينية تعديل مصدري - تعديل التيرّهينية (بالإنجليزية: Tyrrhenian أو Tyrsenian، من اليونانية (الأتيكية: Τυῤῥηνοί Turrhēnoi، الآيونية: Τυρσηνοί Tursēnoi)) هي عائلة لغات منقرضة مقترحة، اقترحها اللساني هلموت ركس في 1998�...