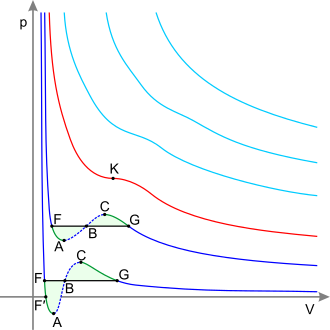
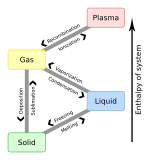
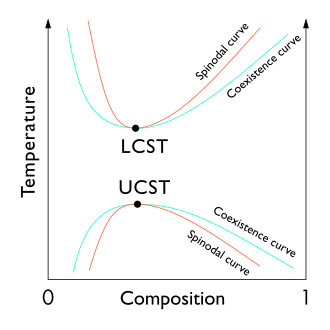Critical point (thermodynamics)
|
Read other articles:

Iklan prangko dengan gambar Charles Nissen pada panel buklet dari prangko PUC Inggris Raya tahun 1929. Pedagang prangko adalah perusahaan atau perorangan yang bergerak di bidang Prangko dan produk filateli. Ini juga termasuk individu yang menjual prangko untuk penggunaan sehari-hari atau meterai pendapatan untuk digunakan pada dokumen pengadilan. Terdapat banyak jenis pedagang prangko yang menjual ke kolektor prangko dan filatelis, dan bisnis mereka berkisar dari operasi rumah kecil hingga pe...

German road cyclist Dominik NerzNerz in 2015Personal informationFull nameDominik NerzBorn (1989-08-25) 25 August 1989 (age 34)Wangen im Allgäu, West GermanyHeight1.80 m (5 ft 11 in)Weight67 kg (148 lb)Team informationCurrent teamRetiredDisciplineRoadRoleRiderRider typeClimberStage racesAmateur teams2008Ista2009Continental Team Milram Professional teams2010Team Milram2011–2012Liquigas–Cannondale2013–2014BMC Racing Team2015–2016Bora–Argon ...

Il governatore dell'Arizona Jan Brewer in un incontro con il presidente Barack Obama nel giugno del 2010 per discutere dell'Arizona SB 1070.[1] Il Support Our Law Enforcement and Safe Neighborhoods Act, ufficialmente Arizona Senate Bill 1070 o in breve Arizona SB 1070, localmente in Arizona semplicemente SB 1070, è una legge dello Stato dell'Arizona negli Stati Uniti d'America che al momento della sua approvazione era la legge più dura sull'immigrazione illegale di tutti gli Stati U...

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (يوليو 2019) موريل سيرف (بالفرنسية: Muriel Cerf) معلومات شخصية اسم الولادة (بالفرنسية: Muriel Gisèle Jacqueline Cerf) الميلاد 4 يونيو 1950 [1][2][3] الدائرة التاسعة في با�...

Pier Dionigi PinelliPier Dionigi PinelliFonctionsDéputéIVe législature du royaume de Sardaigne20 décembre 1849 - 20 novembre 1853DéputéIIIe législature du royaume de Sardaigne30 juillet - 20 novembre 1849Ministre de l'Intérieur du royaume de Sardaigne27 mars - 20 octobre 1849Urbano RattazziGiovanni Filippo GalvagnoDéputéIIe législature du royaume de Sardaigne1er février - 30 mars 1849Ministre de l'Intérieur du royaume de Sardaigne15 août - 16 décembre 1848Giacomo Plezza (en)Ric...

For the 1961 book by Joy Adamson, see Elsa the Lioness. 1972 British filmLiving FreeDVD PosterDirected byJack CoufferWritten byJoy Adamson (Book)Millard Kaufman (Screenplay by)Produced byPaul B. RadinStarring Nigel Davenport Susan Hampshire Geoffrey Keen Edward Judd Peter Lukoye CinematographyWolfgang SuschitzkyEdited byDon DeaconMusic bySol KaplanProductioncompaniesColumbia Pictures CorporationColumbia PicturesOpen Road FilmsHighroadDistributed byColumbia-Warner DistributorsColumbia Pictures...

Cloud collaboration service Not to be confused with air table. Formagrid, Inc.Type of siteCollaborative softwareFounded2012HeadquartersSan Francisco, California, USFounder(s)Howie LiuAndrew OfstadEmmett NicholasIndustryInternetURLairtable.comRegistrationRequiredCurrent statusActive Airtable is a cloud collaboration service headquartered in San Francisco. It was founded in 2012 by Howie Liu, Andrew Ofstad, and Emmett Nicholas. Airtable is a spreadsheet-database hybrid, with the features o...

Cet article est une ébauche concernant une localité indonésienne. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Pekanbaru Montage of Pekanbaru.jpg Noms Nom Indonésien Bandar Raya Pekanbaru Nom Jawi ڤكنبارو Administration Pays Indonésie Province d'Indonésie Riau Maire H. Firdaus, ST, MT Démographie Population 950 571 hab. (2013[1]) Densité 1 503 hab./km2 Géographie Coordonné...

Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. Cet article ne cite pas suffisamment ses sources (juillet 2008). Si vous disposez d'ouvrages ou d'articles de référence ou si vous connaissez des sites web de qualité traitant du thème abordé ici, merci de compléter l'article en donnant les références utiles à sa vérifiabilité et en les liant à la section « Notes et références ». En pratique : Quelles sources sont attendues ? C...

Mahan-class destroyer For other ships with the same name, see USS Tucker. USS Tucker off the Norfolk Navy Yard, Portsmouth, Virginia, 2 March 1937 History United States NameTucker NamesakeSamuel Tucker BuilderNorfolk Navy Yard Laid down15 August 1934 Launched26 February 1936 Commissioned23 July 1936 Stricken2 December 1944 FateStruck mine off Espiritu Santo, New Hebrides, 4 August 1942 General characteristics (as built) Class and typeMahan-class destroyer Displacement 1,500 long ton...

Swedish scenic and costume designer (1928–2022) You can help expand this article with text translated from the corresponding article in Swedish. (February 2021) Click [show] for important translation instructions. Machine translation, like DeepL or Google Translate, is a useful starting point for translations, but translators must revise errors as necessary and confirm that the translation is accurate, rather than simply copy-pasting machine-translated text into the English Wikipedia. ...

Brooks Robinson, the all-time leader in career putouts by a third baseman In baseball statistics, a putout (denoted by PO or fly out when appropriate) is given to a defensive player who records an out by tagging a runner with the ball when he is not touching a base, catching a batted or thrown ball and tagging a base to put out a batter or runner (a force out), catching a thrown ball and tagging a base to record an out on an appeal play, catching a third strike (a strikeout), catching a batt...

Cet article est une ébauche concernant une localité russe. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Seversk (ru) Северск Héraldique Drapeau Rue Kourtchatov à Seversk. Administration Pays Russie Région économique Sibérie de l'Ouest District fédéral Sibérien Sujet fédéral Oblast de Tomsk Code postal 636000 Code OKATO 69 541 Indicatif (+7) 3823 Démographie Population 113 340 hab...

Centre pénitentiaire de Vendin-le-Vieil Les miradors du centre pénitentiaire lors de la construction de l'établissement en 2013. Localisation Pays France Région Hauts-de-France Département Pas-de-Calais DISP Lille Coordonnées 50° 27′ 21″ nord, 2° 49′ 15″ est Géolocalisation sur la carte : Pas-de-Calais Centre pénitentiaire de Vendin-le-Vieil Géolocalisation sur la carte : Hauts-de-France Centre pénitentiaire de Vendin-le-Vieil Géol...

بطولة باوليستا 1974 تفاصيل الموسم بطولة باوليستا البلد البرازيل البطل نادي بالميراس عدد المشاركين 21 بطولة باوليستا 1973 بطولة باوليستا 1975 تعديل مصدري - تعديل بطولة باوليستا 1974 هو موسم من بطولة باوليستا. أشرف على تنظيمه Federação Paulista de Futebol [الإنجليزية]&...

FauguerollescomuneFauguerolles – Veduta LocalizzazioneStato Francia Regione Nuova Aquitania Dipartimento Lot e Garonna ArrondissementMarmande CantoneMarmande-2 TerritorioCoordinate44°26′N 0°15′E44°26′N, 0°15′E (Fauguerolles) Superficie6,92 km² Abitanti655[1] (2009) Densità94,65 ab./km² Altre informazioniCod. postale47400 Fuso orarioUTC+1 Codice INSEE47094 CartografiaFauguerolles Sito istituzionaleModifica dati su Wikidata · Manuale Faugueroll...

Style of rhythm and blues music For other uses, see Doo Wop (disambiguation). Doo-wopFrankie Lymon and the TeenagersStylistic originsRhythm and blues[1]Cultural origins1940s–1950s, African American communities across some major cities on the East CoastDerivative formsBeach musicbeatBrill Buildingpop rockpower popsoulvocal surfRegional scenesNew York CityPhiladelphiaChicagoBaltimoreLos AngelesCincinnatiPittsburghOther topics50s chord progression Doo-wop (also spelled doowop and doo w...

Irish political party Labour Party Páirtí an Lucht OibreLeaderIvana BacikSeanad leaderRebecca Moynihan[1]Parliamentary Party ChairpersonSeán SherlockChairpersonLisa ConnellGeneral SecretaryBillie SparksFounders James Connolly James Larkin William O'Brien Founded28 May 1912; 112 years ago (28 May 1912)Headquarters2 Whitefriars, Aungier Street, DublinYouth wingLabour YouthWomen's wingLabour WomenLGBT wingLabour LGBTMembership (2020)~3,000[2][needs update]...

Square in the City of Westminster, England Not to be confused with Little Dean's Yard or Dean's Park. 51°29′57″N 0°7′45″W / 51.49917°N 0.12917°W / 51.49917; -0.12917 This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Dean's Yard – news · newspapers · books · scholar · JSTOR (...

КоммунаФерьер-сюр-СишонFerrières-sur-Sichon Герб 46°01′34″ с. ш. 3°38′59″ в. д.HGЯO Страна Франция Регион Овернь Департамент Алье Кантон Ле-Мейе-де-Монтань Мэр Jean-Marcel Lazzerini(2008–2014) История и география Площадь 38,58 км² Высота центра 397–980 м Часовой пояс UTC+1:00, летом UTC+2:00 Населе�...