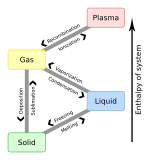
Chemical ionization
|
Read other articles:

Nemanja Radonjić oleh Dmitry Pukalik 2017Informasi pribadiNama lengkap Nemanja RadonjićTanggal lahir 15 Februari 1996 (umur 28)Tempat lahir Niš, FR YugoslaviaTinggi 185 cm (6 ft 1 in)Posisi bermain PenyerangInformasi klubKlub saat ini Red Star BelgradeNomor 49Karier senior*Tahun Tim Tampil (Gol)2017 – Red Star Belgrade 28 (5)Tim nasional2017 – Serbia 5 (0) * Penampilan dan gol di klub senior hanya dihitung dari liga domestik Nemanja Radonjić (lahir 15 Februari 199...

Bopyridae Klasifikasi ilmiah Kerajaan: Animalia Filum: Arthropoda Subfilum: Crustacea Kelas: Malacostraca Ordo: Isopoda Subordo: Cymothoida Superfamili: Bopyroidea Famili: BopyridaeRafinesque, 1815 Genera lihat teks Udang yang memiliki tonjolan menunjukkan parasitisme bopyridae. Bopyridae adalah famili dari krustasea isopoda dalam subordo Cymothoida.[1] Anggota dari famili ini adalah ektoparasit dari kepiting dan udang. Bopyridae hidup di rongga insang atau di bawah karapas, di mana ...

Voce principale: Marina Militare (Italia). Corpo di commissariato militare marittimoStemma della Marina Descrizione generaleNazione Italia ServizioMinistero della difesa, Marina Militare TipoMarina Militare RuoloCorpo della Marina PatronoSanta Barbara, patrona della Marina Militare SimboliBandiera Bandiera di bompresso- Voci su marine militari presenti su Wikipedia Il Corpo di commissariato militare marittimo (abbreviato CM) è un corpo tecnico composto da soli ufficiali della Marina Mi...

PemberitahuanTemplat ini mendeteksi bahwa artikel bahasa ini masih belum dinilai kualitasnya oleh ProyekWiki Bahasa dan ProyekWiki terkait dengan subjek. Perhatian: untuk penilai, halaman pembicaraan artikel ini telah diisi sehingga penilaian akan berkonflik dengan isi sebelumnya. Harap salin kode dibawah ini sebelum menilai. {{PW Bahasa|importance=|class=}} Terjadi [[false positive]]? Silakan laporkan kesalahan ini. 13.55, Jumat, 29 Maret, 2024 (UTC) • hapus singgahan Seban...

Italian Cardinal, philosopher and theologian This biography of a living person needs additional citations for verification. Please help by adding reliable sources. Contentious material about living persons that is unsourced or poorly sourced must be removed immediately from the article and its talk page, especially if potentially libelous.Find sources: Angelo Scola – news · newspapers · books · scholar · JSTOR (April 2019) (Learn how and when to remove...

International Media DistributionCompany typeSubsidiaryIndustryEthnic mediaBroadcastingFounded1998; 26 years ago (1998)HeadquartersCentennial, Colorado, United StatesServicesBroadcastingMarketingParentNBCUniversal Media GroupWebsitewww.imediadistribution.com International Media Distribution (IMD) (formerly International Networks), a division of NBCUniversal, is a leading provider of in-language networks which facilitates the distribution of Asian, European, Middle Eastern and...

Cet article est une ébauche concernant une personnalité moldave et une chanteuse. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Geta BurlacuGeta Burlacu au Concours Eurovision en 2008.BiographieNaissance 22 juillet 1974 (49 ans)BălțiNationalité moldaveActivités Chanteuse, musicienne de jazzPériode d'activité depuis 1993Autres informationsTessiture ContraltoInstrument ViolonGenre artistique JazzSit...

Pour les articles homonymes, voir Megalomys desmarestii. Pilori de la région de la Dalécarlie, Suède (au musée nordique de Stockholm). Un pilori est un poteau ou un dispositif vertical auquel un condamné est attaché temporairement pour être vu et conspué par la foule. Il est censé punir le coupable, impressionner le public, et dissuader d'éventuels criminels, qui craindront de se retrouver un jour en pareille situation. Étymologie L'étymologie la plus vraisemblable qualifie le mo...

The DealDVD promotionSutradaraSteven SchachterProduserIrene LitinskyKeri NakamotoMichael PrupasDitulis olehWilliam H. MacySteven SchachterBerdasarkanThe Dealoleh Peter LefcourtPemeranWilliam H. Macy Meg RyanJason RitterElliott GouldLL Cool JPenata musikJeff BealSinematograferPaul SarossyPenyuntingMatt FriedmanSusan MaggiPerusahaanproduksiMuse Entertainment EnterprisesDistributorPeace Arch EntertainmentTanggal rilis 22 Januari 2008 (2008-01-22) (Sundance Film Festival) Durasi100...

This article uses bare URLs, which are uninformative and vulnerable to link rot. Please consider converting them to full citations to ensure the article remains verifiable and maintains a consistent citation style. Several templates and tools are available to assist in formatting, such as reFill (documentation) and Citation bot (documentation). (August 2022) (Learn how and when to remove this message) Alumni Stadium, home of the Boston College Eagles Fall River, Massachusetts native, Bert Pa...

Fes redirects here. For other uses, see FES. City in Fès-Meknès, MoroccoFez فاسCityFrom the top down: Al-Qarawiyyin Mosque/University, gates of the Royal Palace, and Fes el Bali a.k.a. the Medina of Fez.FezLocation of Fez within MoroccoShow map of MoroccoFezFez (Africa)Show map of AfricaCoordinates: (1,100,000) 34°02′36″N 05°00′12″W / 34.04333°N 5.00333°W / 34.04333; -5.00333Country MoroccoRegionFès-MeknèsFounded789Founded byIdrisid dynastyGover...

Ethnic group in Russia Ethnic group Murmansk FinnsTotal population273LanguagesRussian, FinnishReligionLutheranismRelated ethnic groupsFinnish people, Ingrian Finns, Kvens Murmansk Finns or Kola Finns (Finnish: Muurmanninsuomalaiset, Kuolansuomalaiset) are a group of Finns who live or lived in Murmansk Oblast. They came to Murmansk around 1860 during the Finnish famine of 1866–68.[1] However, there was another immigration period in 1900, due to the building of the Kirov Railway. In 2...

Collection of star charts from antiquity This article contains special characters. Without proper rendering support, you may see question marks, boxes, or other symbols. Star list with distance information, Uruk (Iraq), 320-150 BC, the list gives each constellation, the number of stars and the distance information to the next constellation in ells. Babylonian astronomy collated earlier observations and divinations into sets of Babylonian star catalogues, during and after the Kassite rule over...

Motif of medieval romance The Green Knight has survived beheading by Gawain and carries his own head in this 14th-century manuscript. The beheading game is a literary trope found in Irish mythology and medieval chivalric romance. The trope consists of a stranger who arrives at a royal court and challenges a hero to an exchange of blows: the hero may decapitate the stranger, but the stranger may then inflict the same wound upon the hero. The supernatural nature of the stranger, which makes th...

Salon beralih ke halaman ini. Untuk salon sebagai alat pengeras suara, lihat Pengeras suara dan Pengeras suara genggam. Lihat entri salon kecantikan di kamus bebas Wiktionary. Interior salon kecantikan Salon kecantikan adalah bentuk usaha yang berhubungan dengan perawatan kosmetika, wajah, dan rambut, baik untuk laki-laki maupun perempuan.[1] Variasi lain dari jenis usaha salon kecantikan adalah salon rambut, dan salon tangan dan kuku (manikur). Ada perbedaan yang jelas antara salon k...

Radio station in Kennewick, Washington For the radio station licensed to Yakima, Washington that held the call sign KJOX at 1390 AM from 2004 to 2012, see KTCR (AM). KJOXKennewick, WashingtonBroadcast areaTri-CitiesFrequency1340 kHzBranding1340 ESPN Tri-CitiesProgrammingFormatSportsAffiliationsESPN RadioWestwood OneOwnershipOwnerStephens Media Group(SMG - Tri-Cities, LLC)HistoryFirst air date1945 (as KPKW)Former call signsKPKW (1945–1962)KGRS (1962–1965)KSMK (1965–1973)KOTY (1973–1988...

Award given by the Critics Choice Association Critics' Choice Movie Award for Best Original Screenplay2023 recipients: Greta Gerwig (left) & Noah Baumbach (right)Awarded forBest Original Screenwriting of a Motion PictureLocationLos Angeles, CaliforniaPresented byCritics Choice AssociationFirst awardedEmma Thompson for Sense and Sensibility (1995)Currently held byGreta Gerwig & Noah Baumbach for Barbie (2023)Websitewww.criticschoice.com The Critics' Choice Movie Award for Best Original...

BAE Systems HawkBAE Hawk T.1 trainer dari Skuadron 208 RAF.TipePesawat Latih militerProdusenHawker Siddeley British Aerospace (1977-1999) BAE Systems (mulai 1999)Terbang perdana1974Pengguna utamaBritania RayaPengguna lainIndonesiaMalaysiaAustraliaHarga satuan£18 Juta (Rp278,67 Miliar) (2003)VarianT-45 Goshawk BAE Systems Hawk adalah jet tempur ringan latih (trainer) produksi BAE Hawk sejak 1974. BAE Hawk adalah sebuah perusahaan dari Britania Raya. Hawk merupakan sebuah pesawat jet latih (tr...

Not to be confused with USS General George M. Randall (AP-115). USS Randall (APA-224), circa in 1945 History United States NameRandall NamesakeRandall County, Texas Orderedas a Type VC2-S-AP5 hull, MCE hull 572[1] BuilderPermanente Metals Corporation, Richmond, California Yard number572[1] Laid down15 September 1944 Launched15 November 1944 Sponsored byMrs. Donald D. Dick Commissioned12 December 1944 Decommissioned6 April 1956 Stricken1 July 1960 Identification Hull symbo...

I. Liga 1935-1936 Competizione Fußball-Bundesliga Sport Calcio Edizione 25ª Organizzatore ÖFB Date dal 31 agosto 1935al 24 maggio 1936 Luogo Austria Partecipanti 12 Cronologia della competizione 1934-35 1936-37 Manuale L'edizione 1935-36 della I. Liga vide la vittoria finale del SK Admira Wien. Capocannoniere del torneo fu Wilhelm Hahnemann del SK Admira Wien con 23 reti. Classifica finale Classifica G V N P GF GS Pt 1 SK Admira Wien 22 17 3 2 77 36 37 2 First Vienna FC 22 1...




![{\displaystyle {\ce {M + CH5+ -> CH4 + [M + H]+}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6fbdf60d76f06803668a5ee2679ecda61dc97de5)


![{\displaystyle {\ce {M + C2H5+ -> [M + C2H5]+}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0414052cdb1ea3506bfafcc6f664ea42da620431)