Ulexite
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Surah ke-1الفاتحة al-FātiḥahPembukaTeks ArabTerjemahan KemenagKlasifikasiMakkiyahNama lainFatihatul Kitab,[1] Ummul Qur'an, Ummul Kitab, as-Sab'ul Masani,[2] al-Kanz,[1] al-Wafiyah,[1] al-Kafiyah,[1] al-Asas,[1] asy-Syafiyah,[3] al-Hamd,[1] as-Shalah,[1] al-Ruqyah,[1] asy-Syukru,[1] ad-Du'au,[1] asy-Syifa,[1] al-Waqiyah,[1]JuzJuz 1, Hizb 1Jumlah ruku1Jumlah ayat7Jumlah kat...

Artikel atau sebagian dari artikel ini mungkin diterjemahkan dari Satyajit Ray di en.wikipedia.org. Isinya masih belum akurat, karena bagian yang diterjemahkan masih perlu diperhalus dan disempurnakan. Jika Anda menguasai bahasa aslinya, harap pertimbangkan untuk menelusuri referensinya dan menyempurnakan terjemahan ini. Anda juga dapat ikut bergotong royong pada ProyekWiki Perbaikan Terjemahan. (Pesan ini dapat dihapus jika terjemahan dirasa sudah cukup tepat. Lihat pula: panduan penerjemaha...

Lima ketua menteri wanita petahana di India— Vasundhara Raje, Mamata Banerjee, dan Mehbooba Mufti Enam belas wanita telah menjabat sebagai ketua menteri dari sebuah negara bagian India. Saat ini, tiga diantaranya sedang menjabat—Vasundhara Raje, Mamata Banerjee, dan Mehbooba Mufti.[1] Dari dua belas negara bagian yang dikepalai oleh seorang ketua menteri perempuan, hanya tiga—Delhi, Tamil Nadu dan Uttar Pradesh—yang telah memiliki dua premier wanita. Daftar kronologi ketua men...

Bob Loughman Weibur Perdana Menteri Vanuatu ke-12PetahanaMulai menjabat 20 April 2020[1]PresidenTallis Obed Moses PendahuluCharlot SalwaiPenggantiPetahanaMenteri PendidikanMasa jabatan23 Maret 2013 – 11 Juni 2015Perdana MenteriMoana Carcasses KalosilJoe Natuman PendahuluSteven KalsakauPenggantiAlfred CarlotWakil Perdana Menteri VanuatuMasa jabatan28 Mei 2018 – 18 Juni 2019Perdana MenteriCharlot SalwaiMenteri Pariwisata, Komersial, Perdagangan, dan Bisnis...

العلاقات البحرينية الجيبوتية البحرين جيبوتي البحرين جيبوتي تعديل مصدري - تعديل العلاقات البحرينية الجيبوتية هي العلاقات الثنائية التي تجمع بين البحرين وجيبوتي.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين: وجه المقا...

2010 song by Kanye West Dark FantasySong by Kanye Westfrom the album My Beautiful Dark Twisted Fantasy Recorded2010Genre Hip hop boom bap[1] Length4:41Label Roc-A-Fella Def Jam Songwriter(s) Kanye West Robert Diggs Ernest Wilson Jeff Bhasker Mike Dean Malik Jones Mike Oldfield Jon Anderson Producer(s) RZA Kanye West No I.D. Jeff Bhasker Mike Dean Dark Fantasy is a song by American hip hop recording artist and producer Kanye West from his fifth studio album, My Beautiful Dark Twisted F...

Cet article est une ébauche concernant le sport et la Belgique. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations du projet sport. Comité olympique et interfédéral belge Sigle COIB Sport(s) représenté(s) Omnisports Création 1906 Président Jean-Michel Saive Siège Bruxelles Site internet Site officiel du COIB modifier Le Comité olympique et interfédéral belge (COIB), en néerlandais : Belgisch Olympisch en Interfederaal ...

Senat BarbadosJenisJenisMajelis tinggi PimpinanPresiden SenatReginald Farley, BLP sejak 15 September 2020 Wakil PresidenH. Elizabeth Thompson, BLP sejak 4 Februari 2022 Pemimpin PemerintahLisa Cummins, BLP KomposisiAnggota21Partai & kursiPemerintah BLP (12) Oposisi Independen (7) DLP (2) PemilihanSistem pemilihanDitunjuk oleh PresidenTempat bersidangRuang sidang SenatKamar Senat BarbadosBridgetown, St. Michael, BarbadosSitus webThe Senate L • BBantu...

2010 British filmNanny McPhee and the Big BangUK theatrical release posterDirected bySusanna WhiteWritten byEmma ThompsonBased onNurse Matildaby Christianna BrandProduced byTim BevanEric FellnerLindsay DoranStarring Emma Thompson Maggie Gyllenhaal Rhys Ifans Maggie Smith CinematographyMike EleyEdited bySim Evan-JonesMusic byJames Newton HowardProductioncompanies StudioCanal Relativity Media Working Title Films Three Strange Angels Distributed byUniversal Pictures (North America and Internati...

Digital camera This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Apple QuickTake – news · newspapers · books · scholar · JSTOR (September 2012) (Learn how and when to remove this template message) QuickTakeTop: QuickTake 100 (150 similar in appearance)Bottom: QuickTake 200OverviewMakerApple Computer (branding)...

American Catholic archbishop (born 1959) His Excellency, The Most ReverendPaul Fitzpatrick RussellArchbishop Auxiliary Bishop of DetroitTitular Archbishop of NoviArchdioceseDetroitAppointedMay 23, 2022InstalledJuly 7, 2022Other post(s)Titular Archbishop of NoviOrdersOrdinationJune 20, 1987by Cardinal Bernard LawConsecrationJune 3, 2016by Sean O'Malley, Allen Vigneron and Leo CushleyPersonal detailsBorn (1959-05-02) May 2, 1959 (age 64)Greenfield, Massachusetts, USPrevious post(...

The Jairus' Daughter window in St Andrew's Church, Epworth, Lincolnshire Detail of the Sower and Reaper window in St Peter and St Paul's Church, Pickering Detail of the King Oswald window in Lancaster Priory Edward Holmes Jewitt (1849–1929)[1] was a Pre-Raphaelite artist working in stained glass and other media. He was one of the two chief designers, along with Carl Almquist, for the Lancaster firm of Shrigley and Hunt, producers of stained-glass windows and church-fittings. In...

King of Kings of Iran and non-Iran Hormizd III𐭠𐭥𐭧𐭥𐭬𐭦𐭣King of Kings of Iran and non-Iran[a]Plate of a Sasanian king hunting lions, most likely Hormizd IIIShahanshah of the Sasanian EmpireReign457 – 459CoronationRayPredecessorYazdegerd IISuccessorPeroz IRegentDenagDied459IssueBalendukhtHouseHouse of SasanFatherYazdegerd IIMotherDenagReligionZoroastrianism Hormizd III (Middle Persian: 𐭠𐭥𐭧𐭥𐭬𐭦𐭣; New Persian: هرمز سوم), was the seventeenth...

Slovak footballer (born 1993) Jakub Vojtuš Vojtuš with Slovakia U21 in 2012Personal informationDate of birth (1993-10-22) 22 October 1993 (age 30)Place of birth Spišská Nová Ves, SlovakiaHeight 1.86 m (6 ft 1 in)Position(s) StrikerTeam informationCurrent team Mumbai CityNumber 9Youth career000–2006 Spišská Nová Ves2006–2009 Žilina2010–2013 Inter Milan2011 → Chievo Verona (loan)Senior career*Years Team Apps (Gls)2009 Žilina 1 (0)2012–2013 Inter Milan 0 (...

San DamianocomuneSan-Damiano San Damiano – Veduta LocalizzazioneStato Francia Regione Corsica DipartimentoAlta Corsica ArrondissementCorte CantoneCasinca-Fiumalto TerritorioCoordinate42°24′45″N 9°24′48″E / 42.4125°N 9.413333°E42.4125; 9.413333 (San Damiano)Coordinate: 42°24′45″N 9°24′48″E / 42.4125°N 9.413333°E42.4125; 9.413333 (San Damiano) Altitudine700 m s.l.m. Superficie5,67 km² Abitanti44[1] (...

Un anno d'amore/E se domanisingolo discograficoGrafica di Romano VitaleArtistaMina Pubblicazionenovembre 1964 GenereMusica leggeraPop EtichettaRi-Fi RFN 16071 ArrangiamentiAugusto Martelli Registrazionemono Velocità di rotazione45 giri Formati7 CertificazioniDischi d'oro[1] Italia (1) Mina - cronologiaSingolo precedenteIo sono quel che sonoTu farai(1964)Singolo successivoSe piangi, se ridiPiù di te(1965) Un anno d'amore/E se domani è il 65° singolo di Mina, pubblica...

Частина серії проФілософіяLeft to right: Plato, Kant, Nietzsche, Buddha, Confucius, AverroesПлатонКантНіцшеБуддаКонфуційАверроес Філософи Епістемологи Естетики Етики Логіки Метафізики Соціально-політичні філософи Традиції Аналітична Арістотелівська Африканська Близькосхідна іранська Буддій�...
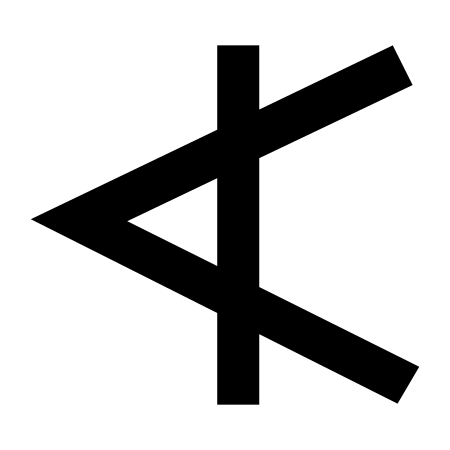
Family of writing systems in ancient Italy This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Old Italic scripts – news · newspapers · books · scholar · JSTOR (August 2021) (Learn how and when to remove this message) Old ItalicAn inscription from the Marsiliana tablet, around 700 BCScript type Alphabet Time per...

Ornaments in sewing This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Trim sewing – news · newspapers · books · scholar · JSTOR (July 2010) (Learn how and when to remove this message) Red fringe trim on a woman's dress c. 1870. Elaborate gold metallic lace trim c. 1760–65. Trim or trimming in clothing a...

City in Oregon, United StatesJohn Day, OregonCityLocation in OregonCoordinates: 44°25′05″N 118°57′23″W / 44.41806°N 118.95639°W / 44.41806; -118.95639CountryUnited StatesStateOregonCountyGrantIncorporated1901Government • MayorRon Lundbom[1]Area[2] • Total2.26 sq mi (5.85 km2) • Land2.26 sq mi (5.85 km2) • Water0.00 sq mi (0.00 km2)Elevation[3 ...

