Triparanol
| |||||||||||||||||||||||||||||||||||||||||
Read other articles:

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada November 2022. Dalam nama Korea ini, nama keluarganya adalah Im. Im Gi-yeongKia Tigers – No. 38PitcherLahir: 16 April 1993 (umur 30)Daegu Bats: Kanan Throws: Kanan KBO debut1 Oktober, 2012, untuk Hanwha Eagles Tim Hanwha Eagles (2012–2014) Ki...
Bukan Sekedar WayangGenreKomediPresenterSuleCepotNegara asalIndonesiaBahasa asliIndonesiaBahasa SundaJmlh. episode577ProduksiProduserBima Indra Sakti Melanie Yoana SihombingDurasi30 menitRumah produksiNET. EntertainmentDistributorIndika GroupRilis asliJaringanNET.Format audioDolby Digital 5.1Rilis23 Juni 2014 (2014-06-23) –31 Juli 2016 (2016-7-31)Acara terkaitCanda Wayang (MNCTV)Asep Show (MNCTV)Wayang Kulit (Indosiar)Cepot Show (MNCTV) Bukan Sekedar Wayang atau BSW adalah s...

Disambiguazione – Se stai cercando altri significati, vedi Gilbert White (disambigua). Questa voce sugli argomenti scienziati britannici e ornitologi è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Gilbert White Gilbert White (Selborne, 18 luglio 1720 – Selborne, 26 giugno 1793) è stato un naturalista e ornitologo britannico. Fra il 1768 e il 1793 collaborò con il naturalista William Markwick, osservando e catalogando più di 400 specie nell'Ham...

American baseball player Baseball player Tom LundstedtCatcherBorn: (1949-04-10) April 10, 1949 (age 75)Davenport, IowaBatted: SwitchThrew: RightMLB debutAugust 31, 1973, for the Chicago CubsLast MLB appearanceSeptember 28, 1975, for the Minnesota TwinsMLB statisticsBatting average.092Home runs0Runs batted in1 Teams Chicago Cubs (1973–74) Minnesota Twins (1975) Thomas Robert Lundstedt (born April 10, 1949), is a former professional baseball player who playe...

Potret Kaisar Guangxu, dengan lukisan Naga Kuning yang dibordir di jubahnya. Naga Kuning (Hanzi Tradisional: 黃龍; Hanzi Sederhana: 黄龙; Pinyin: Huánglóng; Alih aksara Yale: Wong4 Lung4; bahasa Jepang: Kōryū or Ōryū; bahasa Korea: Hwang-Ryong; bahasa Vietnam: Hoàng Long) adalah titisan Dewa Kuning berwujud hewan, pusat alam semesta dalam kepercayaan tradisional Tionghoa dan mitologi Tiongkok.[1] Kaisar Kuning atau...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada April 2017. Osvaldo José Martins JúniorInformasi pribadiTanggal lahir 7 Juli 1982 (umur 41)Tempat lahir BrasilPosisi bermain GelandangKarier senior*Tahun Tim Tampil (Gol)2002 Shimizu S-Pulse 2003 Ventforet Kofu 2003 Sagan Tosu * Penampilan dan gol di klub sen...

1999 video gameAge of WondersDeveloper(s)Triumph Studios, Epic MegaGamesPublisher(s)NA: Gathering of DevelopersEU: Take-Two Interactive[3]Director(s)Lennart SasDesigner(s)Lennart Sas, Arno van WingerdenProgrammer(s)Arno van WingerdenArtist(s)Thomas Cardin, Roy Postma, Lennart SasWriter(s)Raymond BinghamComposer(s)Michiel van den BosSeriesAge of WondersPlatform(s)Microsoft WindowsReleaseNA: November 11, 1999[2]EU: February 9, 2000[1]Genre(s)Turn-based strategyMode(s)Sin...
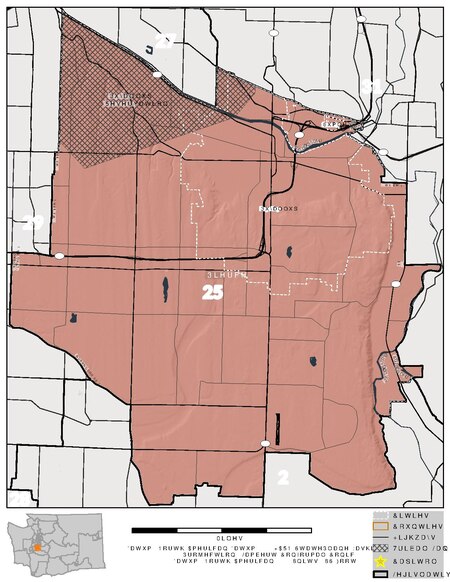
Map of Washington's 25th legislative district Washington's 25th legislative district is one of forty-nine districts in Washington state for representation in the state legislature. The district includes Puyallup and the surrounding area in Pierce County.[1] The district's legislators are state senator Chris Gildon and state representatives Kelly Chambers (position 1) and Cyndy Jacobsen (position 2), all Republicans. See also Washington Redistricting Commission Washington State Legisl...

1993 single by Beth Nielsen Chapman In the Time It TakesSingle by Beth Nielsen Chapman featuring Paul Carrackfrom the album You Hold the Key ReleasedAugust 20, 1993 (1993-08-20)[1]Recorded1993GenreAdult contemporaryLength4:17LabelRepriseSongwriter(s)Beth Nielsen Chapman[2]Producer(s)Jim Ed NormanBeth Nielsen Chapman singles chronology The Moment You Were Mine (1993) In the Time It Takes (1993) Sand and Water (1997) Paul Carrack singles chronology Don't D...

Legal and military structure in medieval Europe This article is about the classic, medieval, Western European form of feudalism. For feudalism in other societies, as well as that of the Europeans, see Examples of feudalism. Investiture of a knight (miniature from the statutes of the Order of the Knot, founded in 1352 by Louis I of Naples) Orava Castle in Slovakia. A medieval castle is a traditional symbol of a feudal society. Feudalism, also known as the feudal system, was a combination of le...

Archaic language in the Vedas (2nd millennium BCE) Not to be confused with the Vedda language. Vedic SanskritNative toPresent-day India, Afghanistan, Nepal and PakistanRegionNorthwestern Indian subcontinentErac. 1500 – 600 BCELanguage familyIndo-European Indo-IranianIndo-AryanVedic SanskritLanguage codesISO 639-3vsnLinguist Listvsn qnk RigvedicGlottologvedi1234IETFsa-vaidikaThis article contains IPA phonetic symbols. Without proper rendering support, you may see question mar...

Not to be confused with The Rolling Stones song You Can't Always Get What You Want. 1984 single by Joe JacksonYou Can't Get What You Want (Till You Know What You Want)Single by Joe Jacksonfrom the album Body and Soul B-sideCha Cha LocoReleased1984Recorded1984StudioMasonic Hall (Manhattan)[1]GenreSophisti-pop, jazzLength3:42 (Single Version) 4:54 (Album Version)LabelA&M RecordsSongwriter(s)Joe JacksonProducer(s)David Kershenbaum and Joe JacksonJoe Jackson singles chronology Memphis...

Beberapa atau seluruh referensi dari artikel ini mungkin tidak dapat dipercaya kebenarannya. Bantulah dengan memberikan referensi yang lebih baik atau dengan memeriksa apakah referensi telah memenuhi syarat sebagai referensi tepercaya. Referensi yang tidak benar dapat dihapus sewaktu-waktu. Psilomelan adalah jenis mineral logam mangan berupa senyawa oksida yang biasanya mengandung logam alkali atau alkali tanah, seperti kalium dan barium.[1] Psilomelan yang sering ditemukan berwarna a...

شعار فرقة الأنياب بالشرطة الجزائرية فرقة الأنياب تم إنشائها للأمن الوطني الجزائري لأول مرة بالمدرسة التطبيقية للشرطة بالصومعة ليتم نقل مقرها فيما بعد إلى الوحدة الجمهورية الثانية للأمن بالدار البيضاء عام 2001 م. عملت الوحدة الجمهورية الثانية للأمن سنوياً ومنذ العام 2003 م ع�...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Idaho Vandals women's basketball – news · newspapers · books · scholar · JSTOR (January 2017) (Learn how and when to remove this message) College basketball team Idaho Vandals women's basketball 2023–24 Idaho Vandals women's basketball team UniversityUniversi...

كارا (بالفرنسية: Kara Mbodj) معلومات شخصية الاسم الكامل سيرين مودو كارا مبودجي الميلاد 11 نوفمبر 1989 (العمر 34 سنة)دياسي، السنغال الطول 1.92 م (6 قدم 3 1⁄2 بوصة) مركز اللعب مدافع / وسط ملعب مدافع الجنسية السنغال معلومات النادي النادي الحالي السيلية الرقم 14 مسيرة الش...

アメリカ陸軍航空軍 創設 1941年6月20日 - 1947年9月17日国籍 アメリカ合衆国軍種 アメリカ陸軍タイプ 陸軍航空隊任務 航空戦上級部隊 アメリカ陸軍主な戦歴 第二次世界大戦表話編歴テンプレートを表示 アメリカ陸軍航空軍(United States Army Air Forces, USAAF)は、かつて存在したアメリカ陸軍の部門。アメリカ空軍の前身である。第二次世界大戦中の1941年に陸軍地上軍と同格�...

この項目では、日本中央競馬会(JRA)の3歳馬による重賞について説明しています。JRAの古馬重賞競走については「京成杯オータムハンデキャップ」を、船橋競馬場の重賞競走については「京成盃グランドマイラーズ」をご覧ください。 京成杯Keisei Hai[1]開催国 日本主催者 日本中央競馬会競馬場 中山競馬場創設 1961年1月15日2024年の情報距離 芝2000m格付け GIII賞金 1�...

Italian footballer Federica Cafferata Personal informationDate of birth (2000-05-07) 7 May 2000 (age 24)Place of birth Genova, ItalyPosition(s) DefenderTeam informationCurrent team JuventusNumber 4Senior career*Years Team Apps (Gls)2016–2021 Napoli 20 (1)2021–2023 Fiorentina 43 (0)2023– Juventus 2 (0)International career2016–2017 Italy U17 6 (2)2022–2023 Italy 2 (0) *Club domestic league appearances and goals, correct as of 25 February 2024 (UTC) Federica Cafferata (born 7 May ...

BlessedBernardo de HoyosS.J.PriestBorn(1711-08-21)21 August 1711Torrelobatón, Valladolid, Kingdom of SpainDied29 November 1735(1735-11-29) (aged 24)Valladolid, Kingdom of SpainVenerated inCatholic ChurchBeatified18 April 2010, Plaza de Colón, Valladolid, Spain by Archbishop Angelo AmatoFeast29 NovemberAttributesPriest's cassock Bernardo Francisco de Hoyos de Seña, SJ (21 August[1] 1711 – 29 November 1735), best known simply as Bernardo de Hoyos, was a Spanish Catholic p...
