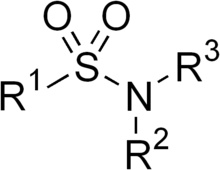
Sulfonamide (medicine)
|
Read other articles:

Alun-alun pusat Estremoz dengan tiang pualam bergaya Manuelin (berawal dari abad ke-16, direstorasi pada abad ke-20. Estremoz (IPA: [(ɨ)ʃtɾɨ.moʃ]) ialah sebuah kotamadya di Portugal dengan wilayah seluas 514 km² dan berpenduduk sekitar 15.064 jiwa. Paroki Arcos Estremoz (Santa Maria) Estremoz (Santo André) Évora Monte (Santa Maria) Glória Santa Vitória do Ameixial Santo Estevão São Bento de Ana Loura São Bento do Ameixial São Bento do Cortiço São Domingos de Ana Loura São Lou...

Ichiki TatsuoIchiki Tatsuo pada 1941Lahir1906 (1906)Prefektur Kumamoto, JepangMeninggal9 Januari 1949(1949-01-09) (umur 42–43)Kabupaten Malang, Negara Jawa Timur, Hindia BelandaDikenal atasMembelot ke Indonesia selama Revolusi Nasional Indonesia Ichiki Tatsuo atau Abdul Rahman (䝢㲳֛) (lahir di kota Taraki, prefektur Kumamoto, selatan Kyushu, Jepang pada 1906[1] - meninggal di Dampit, Malang, Jawa Timur, Indonesia pada 9 Januari 1949) adalah seorang pembelot Jepang yan...

Chronologies Données clés 1887 1888 1889 1890 1891 1892 1893Décennies :1860 1870 1880 1890 1900 1910 1920Siècles :XVIIe XVIIIe XIXe XXe XXIeMillénaires :-Ier Ier IIe IIIe Chronologies géographiques Afrique Afrique du Sud, Algérie, Angola, Bénin, Botswana, Burkina Faso, Burundi, Cameroun, Cap-Vert, République centrafricaine, Comores, République du Congo, République démocratique du Congo, Côte d'Ivoire, Djibouti, Égyp...

Palm adalah Personal Digital Assistant (PDA) yang menjalankan Palm OS. Palm TX menawarkan kemampuan untuk browsing Internet nirkabel Model awal—PalmPilot Personal Berkas:Palm 3xe with portable keyboard on top of original manual handbook etc with custom franklin dictionary case (promotional) and go-vox voice recorder fliplid.jpgUnit Palm IIIxe dengan Aksesoris. Palm IIIc adalah versi pertama Palm dengan layar berwarna Palm m100 monokrom PalmOne Tungsten, versi T5 yang terkenal sebagai sukses...

This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article relies largely or entirely on a single source. Please help improve this article by introducing citations to additional sources.Find sources: European University of Rome – news · newspapers · books · scholar · JSTOR (November 2023) This article relies excessively on references to primary s...

Untuk kegunaan lain, lihat Rumah Fallingwater (disambiguasi). FallingwaterU.S. National Historic LandmarkPennsylvania state historical markerLetak:Mill Run, PennsylvaniaKota terdekat:UniontownKoordinat:39°54′22″N 79°28′5″W / 39.90611°N 79.46806°W / 39.90611; -79.46806Koordinat: 39°54′22″N 79°28′5″W / 39.90611°N 79.46806°W / 39.90611; -79.46806Dibangun:1936–1939Arsitek:Frank Lloyd WrightGaya arsitektur:Arsitektur Mo...

For the anthropologist, see Tim Trench (anthropologist). Comics character Tim TrenchPublication informationPublisherDC ComicsFirst appearanceWonder Woman v1 #179 (November–December 1968)Created byDenny O'NeilIn-story informationFull nameTimothy TrenchTeam affiliationsHero HotlineThe Croatoan Society Tim Trench is a character in the DC Comics universe, who first appeared in Wonder Woman v1 #179 (November–December 1968).[1] He was later killed in 52 Week 18 (September 2006). Fiction...

Disambiguazione – Se stai cercando il politico, vedi Ludovico D'Aragona. Ludovico di SiciliaRe di TrinacriaStemma In carica15 agosto 1342 -16 ottobre 1355 PredecessorePietro II SuccessoreFederico IV Nome completoLudovico (o Luigi) NascitaCatania, 1335 o 1337 MorteCastello di Aci, 16 ottobre 1355 SepolturaCattedrale di Sant'Agata, Catania Casa realeAragona di Sicilia PadrePietro II MadreElisabetta di Carinzia FigliAntonio eLuigi, naturali ReligioneCattolicesimo Ludovico (o Luigi) d'Ar...

هذه مقالة غير مراجعة. ينبغي أن يزال هذا القالب بعد أن يراجعها محرر؛ إذا لزم الأمر فيجب أن توسم المقالة بقوالب الصيانة المناسبة. يمكن أيضاً تقديم طلب لمراجعة المقالة في الصفحة المخصصة لذلك. (أكتوبر 2023) تحوي هذه المقالة أو هذا القسم ترجمة آلية. فضلًا، ساهم في تدقيقها وتحسينها �...

City in Razavi Khorasan province, Iran For the administrative division, see Rud Ab District. For other places with a similar name, see Rud Ab. City in Razavi Khorasan, IranRud Ab Persian: رودابCityRud AbCoordinates: 36°01′15″N 57°18′46″E / 36.02083°N 57.31278°E / 36.02083; 57.31278[1]CountryIranProvinceRazavi KhorasanCountySabzevarDistrictRud AbPopulation (2016)[2] • Total4,028Time zoneUTC+3:30 (IRST)Rud Ab at GEOnet Na...

Gunboat of the United States Navy History Spain NamePampanga BuilderManila Ship Company, Cavite, Philippines Laid downMarch 1887 LaunchedFebruary 1888 FateCaptured by US Army, Manila Bay, June 1898 United States NameUSS Pampanga Acquiredby capture, June 1898 Commissioned9 November 1899 Decommissioned18 June 1902 Recommissioned30 January 1904 Decommissioned30 April 1907 Recommissioned12 April 1911 Decommissioned31 May 1915 Recommissioned3 January 1916 Decommissioned6 November 1928 FateSunk by ...

この記事は検証可能な参考文献や出典が全く示されていないか、不十分です。出典を追加して記事の信頼性向上にご協力ください。(このテンプレートの使い方)出典検索?: コルク – ニュース · 書籍 · スカラー · CiNii · J-STAGE · NDL · dlib.jp · ジャパンサーチ · TWL(2017年4月) コルクを打ち抜いて作った瓶の栓 コルク(木栓、�...

Bilateral relationsAlbanian–Spanish relations Spain Albania Formal relations between Albania and Spain were established in 1986. Albania has an embassy in Madrid, and Spain has an embassy in Tirana. The countries are both members of the North Atlantic Treaty Organization, the Organization for Security and Co-operation in Europe, and the Union for the Mediterranean. Spain does not recognize Kosovo as a sovereign state, which has led Albania to distance itself. Spain is neutral on Albania's E...

Protected area in Romania This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Măcin Mountains – news · newspapers · books · scholar · JSTOR (January 2017) (Learn how and when to remove this message)The Danube and Măcin Mountains in the background The Măcin Mountains (Romanian: Munții Măcin) is a mountain ra...

Кинематограф Туркменистана — киноискусство и киноиндустрия Туркменистане. Уполномоченным государственным органом в области кинематографии является Министерство культуры Туркменистана. Основной производящей кинокомпанией в Туркменистане является объединение «�...

Viking 2Immagine del veicolo Dati della missioneOperatoreNASA NSSDC ID1975-083A SCN08199 DestinazioneMarte Esito Missione compiuta con successo L'orbiter è tuttora in orbita attorno a Marte Il lander ha operato per quasi 5 anni VettoreLanciatore Titan IIIE-Centaur Lancio9 settembre 1975, 18:39 UTCLaunch Pad 41, Cape Canaveral, Florida Luogo lancioCape Canaveral Air Force Station Space Launch Complex 41 Atterraggio3 settembre 1976 Sito atterraggioMarte Proprietà del veicolo spazialeMass...

2016年美國總統選舉 ← 2012 2016年11月8日 2020 → 538個選舉人團席位獲勝需270票民意調查投票率55.7%[1][2] ▲ 0.8 % 获提名人 唐納·川普 希拉莉·克林頓 政党 共和黨 民主党 家鄉州 紐約州 紐約州 竞选搭档 迈克·彭斯 蒂姆·凱恩 选举人票 304[3][4][註 1] 227[5] 胜出州/省 30 + 緬-2 20 + DC 民選得票 62,984,828[6] 65,853,514[6]...

В Википедии есть статьи о других людях с фамилией Смит.Фанни Кокрейн Смитангл. Fanny Cochrane Smith Дата рождения декабрь 1834 Место рождения Флиндерс, Тасмания, Австралия Дата смерти 24 февраля 1905(1905-02-24) (70 лет) Место смерти Port Cygnet[вд], Тасмания, Австралия Страна Австралия Фан�...

Part of a series onEthnicity in Boston African American Albanian Brazilian Cape Verdean Chinese Hispanics and Latinos Dominican French Germans Irish Italian Jewish Korean Native Americans Portuguese Puerto Rican Vietnamese vte The paifang gate to Boston's Chinatown Kam Man Food in Quincy, Massachusetts The Boston metropolitan area has an active Chinese American community. As of 2013, the Boston Chinatown was the third largest Chinatown in the United States, and there are also Chinese populat...
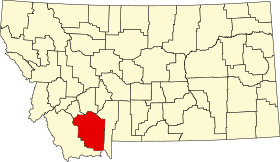
لمعانٍ أخرى، طالع مقاطعة ماديسون (توضيح). مقاطعة ماديسون الإحداثيات 45°18′N 111°55′W / 45.3°N 111.92°W / 45.3; -111.92 [1] تاريخ التأسيس 1865 سبب التسمية جيمس ماديسون تقسيم إداري البلد الولايات المتحدة[2] التقسيم الأعلى مونتانا العاصمة في...