Spinosad
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Cesare Beccaria Cesare Bonesano de Beccaria atau Cesare Beccaria (15 Maret 1738 – 28 November 1794) adalah seorang ahli hukum filsuf yang bersala dari Italia yang terkenal dengan bukunya Dei delitti e delle pene atau dalam versi Inggrisnya On Crimes and Punishments, berisikan tentang kejahatan-kejahatan dan hukuman-hukuman pada tahun 1974.[1] Beccaria menolak teori hukuman-menakutkan, dan berpendirian bahwa negara berhak menghukum seorang yang melakukan kejahatan.[...

Untuk tempat lain yang bernama sama, lihat Benteng (disambiguasi). BentengKecamatanPeta Kecamatan BentengBentengPeta Kepulauan SelayarKoordinat: 6°07′13″S 120°27′39″E / 6.1202026°S 120.4608986°E / -6.1202026; 120.4608986Negara IndonesiaProvinsiSulawesi SelatanKabupatenKepulauan SelayarPemerintahan • CamatAndi Mastatar, S.PdiLuas • Total7,12 km2 (2,75 sq mi)Populasi (2010[1][2]) • T...

Cet article est une ébauche concernant une chaîne de télévision et l’Allemagne. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. ProSieben FunCaractéristiquesCréation 3 mai 2012Propriétaire ProSiebenSat.1 MediaFormat d'image 576i (SDTV), 720p (HDTV)Langue AllemandPays AllemagneStatut Généraliste nationale privéeSiège social UnterföhringSite web www.prosiebenfun.deDiffusionDiffusion Satellite, câble...

This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) You can help expand this article with text translated from the corresponding article in French. (May 2020) Click [show] for important translation instructions. Machine translation, like DeepL or Google Translate, is a useful starting point for translations, but translators must revise errors as necessary and confirm that the translatio...

Stasiun Citayam B22 Penampakan Stasiun Citayam, 2021Nama lainStasiun CitayemLokasiJalan Raya Citayam[1]Bojong Pondok Terong, Cipayung, Depok, Jawa Barat 16444IndonesiaKoordinat6°26′42″S 106°47′59″E / 6.44500°S 106.79972°E / -6.44500; 106.79972Koordinat: 6°26′42″S 106°47′59″E / 6.44500°S 106.79972°E / -6.44500; 106.79972Ketinggian+120 mOperator KAI Commuter Letak km 37+760 lintas Jakarta-Manggarai-Bogor km 37+810 l...

American film director, special effects designer (1942–2022) This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Douglas Trumbull – news · newspapers · books · scholar · JSTOR (June 2011) (Learn how and when to remove this message) Douglas TrumbullTrumbull at the annual FMX Conference in 2012BornDouglas Hunt T...

「俄亥俄」重定向至此。关于其他用法,请见「俄亥俄 (消歧义)」。 俄亥俄州 美國联邦州State of Ohio 州旗州徽綽號:七葉果之州地图中高亮部分为俄亥俄州坐标:38°27'N-41°58'N, 80°32'W-84°49'W国家 美國加入聯邦1803年3月1日,在1953年8月7日追溯頒定(第17个加入联邦)首府哥倫布(及最大城市)政府 • 州长(英语:List of Governors of {{{Name}}}]]) •&...

Renault R.S.18R.S.18 yang dikemudikan oleh Nico Hülkenberg pada Grand Prix Austria 2018.KategoriFormula SatuKonstruktorRenaultPerancangBob Bell (Direktur Teknis)Nick Chester (Direktur Teknis Sasis) Naoki Tokunaga (Kepala Transformasi) Chris Cooney (Direktur Mekanis) Martin Tolliday (Kepala Perancang)Simon Virrill (Pimpinan Proyek) Pete Machin (Kepala Aerodinamika)PendahuluRenault R.S.17PenerusRenault R.S.19Spesifikasi teknis[1][2][3]SasisCetakan monocoque serat karbon...
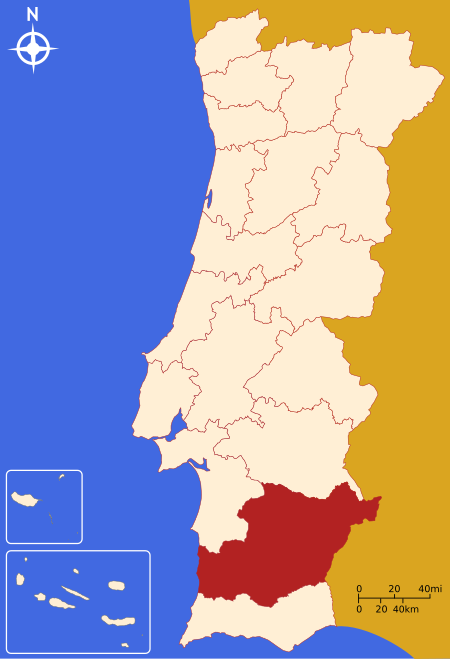
District of Portugal For other uses, see Beja (disambiguation). District in Alentejo, PortugalDistrict of BejaDistrict FlagCoat of armsCountryPortugalRegionAlentejoHistorical provinceBaixo AlentejoNo. of municipalities14No. of parishes75CapitalBejaArea • Total10,225 km2 (3,948 sq mi)Population (2011) • Total152,758 • Density15/km2 (39/sq mi)ISO 3166 codePT-02No. of parliamentary representatives3 The Beja District (Portuguese pronunc...

Studies and methods used by scholars to develop a history of Britain's empire The historiography of the British Empire refers to the studies, sources, critical methods and interpretations used by scholars to develop a history of the British Empire. Historians and their ideas are the main focus here; specific lands and historical dates and episodes are covered in the article on the British Empire. Scholars have long studied the Empire, looking at the causes for its formation, its relations to ...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Spaniards in Mexico – news · newspapers · books · scholar · JSTOR (July 2022) (Learn how and when to remove this message) Ethnic group Spaniards in MexicoEspañoles en MéxicoFagoaga Arozqueta Basque family who migrated to Mexico City, c. 1735Total population20...

Denzel Washington nel 2018 Oscar al miglior attore non protagonista 1990 Oscar al miglior attore 2002 Denzel Hayes Washington Jr. (Mount Vernon, 28 dicembre 1954) è un attore, regista e produttore cinematografico statunitense. Ha ricevuto molti riconoscimenti per il suo lavoro cinematografico fin dagli anni ottanta, incluse le sue interpretazioni di personaggi come l'attivista anti-apartheid sudafricano Steve Biko in Grido di libertà (1987), l'attivista per i diritti umani Malcolm X nel fil...

سلافيانسك-نا-كوباني علم شعار الاسم الرسمي (بالروسية: Славянская) الإحداثيات 45°15′31″N 38°07′29″E / 45.258611111111°N 38.124722222222°E / 45.258611111111; 38.124722222222 تاريخ التأسيس 1865 تقسيم إداري البلد روسيا[2][1] التقسيم الأعلى كراسنودار كراي (26 أكتوبر 1965...

جنيح الطائرة aileron الجنيح أو Aileron هو سطح توجيه مفصلي متصل بالحافة الخلفية لجناحي الطائرة. فهو يستخدم لدوران الطائرة. والجنيحان مرتبطان ببعضهما البعض بشكل مثالي بحيث احدهما يرتفع إذا انخفض الآخر، فالجناح الذي به الجنيح المنخفض تزداد عنده قوة الرفع فيرتفع بينما الجناح الآخر �...

1967 political statement by Tanzanian President Julius Nyerere For other uses, see Arusha (disambiguation). This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Arusha Declaration – news · newspapers · books · scholar · JSTOR (May 2022) (Learn how and when to remove this message) Arusha Declaration Monument The A...

Voce principale: Associazione Calcio Monza Brianza 1912. Associazione Sportiva Simmenthal-MonzaStagione 1960-1961Sport calcio Squadra Simmenthal-Monza Allenatore Hugo Lamanna Presidente Claudio Sada Serie B5º Maggiori presenzeCampionato: Livio Ghioni (38) Miglior marcatoreCampionato: Maschietto (6) StadioCittà di Monza 1959-1960 1961-1962 Si invita a seguire il modello di voce Questa pagina raccoglie le informazioni riguardanti l'Associazione Sportiva Simmenthal-Monza nelle competizio...

This biography of a living person needs additional citations for verification. Please help by adding reliable sources. Contentious material about living persons that is unsourced or poorly sourced must be removed immediately from the article and its talk page, especially if potentially libelous.Find sources: Andy Moor producer – news · newspapers · books · scholar · JSTOR (February 2017) (Learn how and when to remove this message) Andy MoorBackgro...
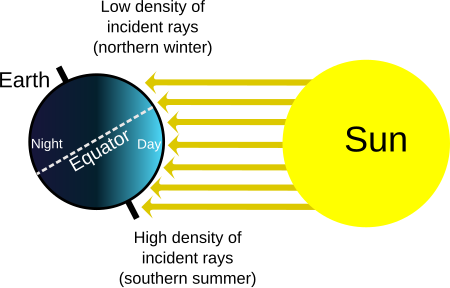
Hottest of the four temperate seasons This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Summer – news · newspapers · books · scholar · JSTOR (November 2023) (Learn how and when to remove this message) Not to be confused with Sumer. Summers redirects here. For other uses, see Summer (disambiguation) and Summer...

Traditional assortment of foods served at Christmas in Finland This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Joulupöytä – news · newspapers · books · scholar · JSTOR (December 2019) (Learn how and when to remove this message) Modern Finnish joulupöytä Joulupöytä (pronounced [ˈjou̯luˌpøy̯tæ&...

ダンカン・アームストロング 泳法 自由形所属 西オーストラリア大学 フロリダ大学生年月日 (1968-04-07) 1968年4月7日(56歳)生誕地 オーストラリア クイーンズランド州ロックハンプトン身長 188 cm体重 74 kg 獲得メダル 男子競泳 オーストラリア オリンピック 金 1988年ソウル 200m自由形 銀 1988年ソウル 400m自由形 コモンウェルスゲームズ 金 1986年エディンバラ 400m自由形 金 1...

