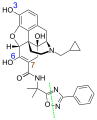Naldemedine
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Apa KabarAlbum studio karya U'CampDirilis24 April 2008Direkam2008GenreAlternative, Rock, Pop, Hip MetalDurasi?LabelNagaswaraProduserU'CampKronologi U'Camp Melangkah (album) (1998)String Module Error: Match not found1998 Apa kabar (2008) Apa Kabar merupakan album musik terkahir hasil karya U'Camp. Dirilis tahun 2008. Daftar lagu Apa Kabar Selingkungkuh Cinta Menanti Jawaban Gila Tapi Waras Preman Ku sudah Bosan Semesta Alam Relakan Izinkan Aku Bayangan lbsU'CampPersonilIram- (gitar) · Dhi...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Januari 2023. Farah Maalim Mohamed Wakil Ketua Majelis Nasional KenyaMasa jabatan15 Januari 2008 – 14 Januari 2013 PendahuluDavid MusilaPenggantiJoyce LabosoAnggota Parlemen dari Daerah Pilih LagderaMasa jabatan15 Januari 2008 – 14 Januari 2013...

In alchemy, using a Philosopher's Stone to transmute a lesser substance into a higher form This article is about the alchemical concept. For other uses, see Projection. Depiction of Sedziwój performing a transmutation for Sigismund III by Jan Matejko, 1867 Projection was the ultimate goal of Western alchemy. Once the philosopher's stone or powder of projection had been created, the process of projection would be used to transmute a lesser substance into a higher form; often lead into gold. T...

Genus of large azhdarchid pterosaur from the Late Cretaceous Not to be confused with Arambourgia. ArambourgianiaTemporal range: Maastrichtian PreꞒ Ꞓ O S D C P T J K Pg N Holotype fossil cast at Museum Histoire Naturelle, Paris Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Chordata Order: †Pterosauria Suborder: †Pterodactyloidea Family: †Azhdarchidae Subfamily: †Quetzalcoatlinae Genus: †ArambourgianiaNessov vide Nessov & Yarkov, 1989 Species: †A....

PT Rekayasa IndustriNama dagangRekindJenisPerseroan terbatasIndustriKonstruksiDidirikan12 Agustus 1981; 42 tahun lalu (1981-08-12)KantorpusatJakarta, IndonesiaWilayah operasiIndonesiaTokohkunciTriyani Utaminingsih[1](Direktur Utama)Ngakan Timur Antara[1](Komisaris Utama)JasaEPCC dan pembangkitan listrikPendapatanRp 7,904 triliun (2019)[2]Laba bersihRp 217,992 milyar (2019)[2]Total asetRp 10,481 triliun (2019)[2]Total ekuitasRp 976,796 milyar (2019)...

Duta Besar Niger untuk IndonesiaPetahanaLeko Adosejak 2019 Berikut adalah daftar duta besar Republik Niger untuk Republik Indonesia. Nama Kredensial Selesai tugas Ref. Leko Ado 20 November 2019 Petahana [1][cat. 1] Catatan ^ Berkedudukan di New Delhi. Lihat pula Daftar Duta Besar Indonesia untuk Niger Daftar duta besar untuk Indonesia Referensi ^ Presiden Jokowi Terima Surat Kepercayaan 14 Duta Besar Negara Sahabat. Kementerian Sekretariat Negara Republik Indonesia. 20 No...

Study of work and workers Vancouver Labour Temple during the general strike in 1918 Labor relations or labor studies is a field of study that can have different meanings depending on the context in which it is used. In an international context, it is a subfield of labor history that studies the human relations with regard to work in its broadest sense and how this connects to questions of social inequality. It explicitly encompasses unregulated, historical, and non-Western forms of labor. Her...

Mobile phone model Samsung E1107ManufacturerSamsungCompatible networksGSM 900, GSM 1800SuccessorSamsung S7550Form factorcandybarDimensions105.2 x 44.15 x 16.4Mass119 gStorage1.5MB (up to 500 contacts)BatteryLi-ion 800 mAhDisplay128x128px, 1.52, 65K CSTNData inputsNumeric keypadOthersolar panel The Samsung E1107 (also known as Crest Solar or Solar Guru) is a mobile phone designed for a rural lower budget market. It was first released in India on July 10, 2009 with an initial price of ₹2,799....

Terms of honor in Judaism This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Honorifics in Judaism – news · newspapers · books · scholar · JSTOR (August 2018) (Learn how and when to remove this message) There are a number of honorifics in Judaism that vary depending on the status of, and the relationship to, t...

Unincorporated community in Ohio, U.S. The Outville Depot, a historic site Outville is an unincorporated community in Licking County, in the U.S. state of Ohio.[1] History Outville had its start when the railroad was extended to that point.[2] A post office was established at Outville in 1858, and remained in operation until 1960.[3] References ^ U.S. Geological Survey Geographic Names Information System: Outville, Ohio ^ Brister, Edwin M. P. (1909). Centennial History...

American politician For the Florida Supreme Court justice, see William Glenn Terrell. Official portrait, c.1850s William Terrell (1786 – July 4, 1855) was elected as a United States representative from Georgia.[1][2] Family See also: Terrell (surname) He was one of two children born to Joel and Lucy (Ragland) Terrell.[2][3] He was born in either Fairfax County[1] (or Louisa County),[2] Virginia. He moved with his parents to Wilkes County, Geor...

Dayr HafirNom officiel (ar) دير حافرNom local (ar) دير حافرGéographiePays SyrieGouvernorat AlepDistrict District de Dayr Hafir (chef-lieu)Sous-district Dayr Hafir Subdistrict (d)Superficie 5,7 km2Altitude 342 mCoordonnées 36° 09′ 33″ N, 37° 42′ 15″ EDémographiePopulation 35 409 hab. (2013)Densité 6 212,1 hab./km2 (2013)FonctionnementStatut Populated place in Syria (d) Géolocalisation sur la carte ...

Historic road in Oregon Barlow RoadRoute of the Barlow Road (red); some consider the yellow route from The Dalles as part of the roadLocationOregon, USANearest cityThe DallesGovernment CampOregon CityEstablished1845Barlow RoadU.S. National Register of Historic PlacesU.S. Historic district NRHP reference No.92000334[1]Added to NRHPApril 13, 1992 The Barlow Road (at inception, Mount Hood Road) is a historic road in what is now the U.S. state of Oregon. It was built in 184...

1998 Indian general election ← 1996 16, 22 and 28 February 1998 1999 → ← outgoing memberselected members →543 of the 545 seats in the Lok Sabha272 seats needed for a majorityRegistered605,880,192Turnout61.97% ( 4.03pp) First party Second party Third party Leader Atal Bihari Vajpayee Sitaram Kesri Harkishan Singh Surjeet Party BJP INC CPI(M) Alliance NDA INC+ LF Last election 20.29%, 161 seats 28.80%, 140 seats 6.12%, 32 seats Sea...

Presidente della Repubblica Ceca Prezident České republikyStendardo presidenziale della Repubblica Ceca Petr Pavel, attuale Presidente della Repubblica Ceca Stato Rep. Ceca TipoCapo di Stato In caricaPetr Pavel da9 marzo 2023 Istituito2 febbraio 1993 PredecessorePresidente della Cecoslovacchia Nominato davoto popolare Durata mandatocinque anni, rinnovabile una volta consecutivamente Bilancio2.235.600 Kč (86830 $)[1] SedeCastello di Praga, Praga Sito webwww.hrad.cz/ Modifi...
本文或本章節是關於未來的公共运输建設或計划。未有可靠来源的臆測內容可能會被移除,現時內容可能與竣工情況有所出入。 此条目讲述中国大陆處於施工或详细规划阶段的工程。设计阶段的資訊,或許与竣工后情況有所出入。无可靠来源供查证的猜测会被移除。 设想中的三条路线方案[1]。 臺灣海峽隧道或臺湾海峡橋隧(英語:Taiwan Strait Tunnel Project)是一项工程�...

The following list is composed of items, techniques and processes that were invented by or discovered by people from Spain. Spain was an important center of knowledge during the medieval era. While most of western and southern Europe suffered from the collapse of the Roman Empire, although declining, some regions of the former empire, Hispania (the Iberian Peninsula), southern Italy, and the remainder of the Eastern Roman Empire or Byzantine Empire, did not to suffer from the full impact of ...

Questa voce o sezione sull'argomento Spagna non cita le fonti necessarie o quelle presenti sono insufficienti. Puoi migliorare questa voce aggiungendo citazioni da fonti attendibili secondo le linee guida sull'uso delle fonti. Comunità Valencianacomunità autonomaComunitat ValencianaComunidad Valenciana (dettagli) Comunità Valenciana – VedutaIl Palau de la Generalitat Valenciana, sede della comunità autonoma LocalizzazioneStato Spagna AmministrazioneCapoluogo Valencia Presiden...

لمعانٍ أخرى، طالع جيمس روبنسون (توضيح). جيمس روبنسون (كاتب بريطاني) معلومات شخصية الميلاد 1 أبريل 1963 (61 سنة)[1][2] مانشستر مواطنة المملكة المتحدة الحياة العملية المواضيع قصص مصورة المهنة فنان قصص مصورة، وكاتب سيناريو، ومخرج أفلام، وكا�...

В Википедии есть статьи о других людях с именем Сигизмунд. Сигизмунд IIIшвед. Sigismund III Якоб Трошель. Портрет короля Польши Сигизмунда III Вазы, 1610-е годы. Королевский замок в Варшаве Король польский и великий князь литовский 18 сентября 1587 — 19 апреля 1632 Девиз Pro jure et populo(За сп�...