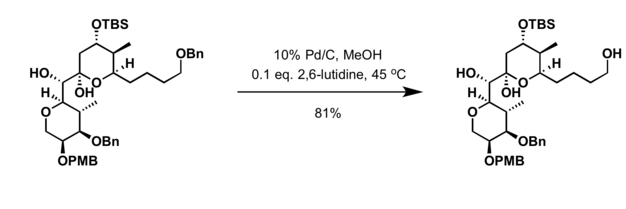
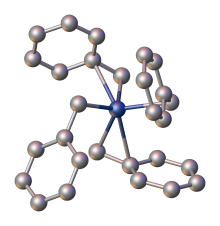
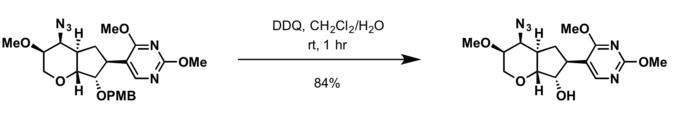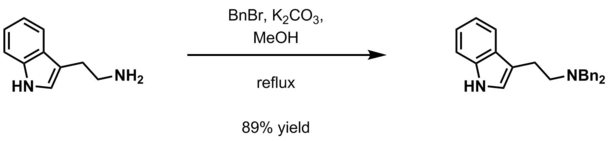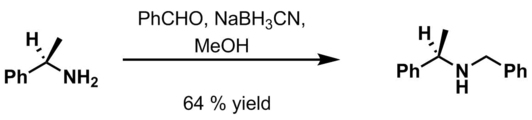Benzyl group
| ||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

George Abela Presiden Malta Ke-8Masa jabatan4 April 2009 – 4 April 2014 PendahuluEdward Fenech AdamiPenggantiMarie Louise Coleiro Preca Informasi pribadiLahir22 April 1948 (umur 75)Qormi, MaltaSunting kotak info • L • B George Abela (lahir 22 April 1948) adalah Presiden Malta saat ini. Ia mulai menjabat sejak 4 April 2009 sebelumnya dia pernah menjabat sebagai presiden timnas Malta dari tahun 1982 hingga 1992. Bajnai terpilih menjadi presiden menggantikan Edward ...

Fictional species of bird This article is about the species from Final Fantasy. For the game series starring the character Chocobo, see Chocobo (series). Fictional character ChocoboFinal Fantasy characterChocobo artwork by Tetsuya Nomura in Final Fantasy VIIFirst gameFinal Fantasy II (1988)Created byKoichi IshiiDesigned byKoichi IshiiToshiyuki Itahana (Chocobo series) The Chocobo (Japanese: チョコボ, Hepburn: Chokobo) is a fictional species created for the Final Fantasy franchise by Squar...

Sonic Advance PublikasiGame Boy AdvanceJP: 20 Desember 2001NA: 3 Februari 2002PAL: 8 Maret 2002N-GageWW: 7 Oktober 2003AndroidJP: 25 November 2011J2MEWW: 2011GenrePlatform, aksiBahasa Daftar Inggris dan Jepang 60 Karakteristik teknisSistem operasiAndroid PlatformGame Boy Advance, N-Gage dan Android Modepermainan video multipemain dan Permainan video pemain tunggal FormatROM cartridge dan unduhan digital Metode inputlayar sentuh Format kode Daftar 30 Informasi pengembangPengembangDimpsSonic Te...
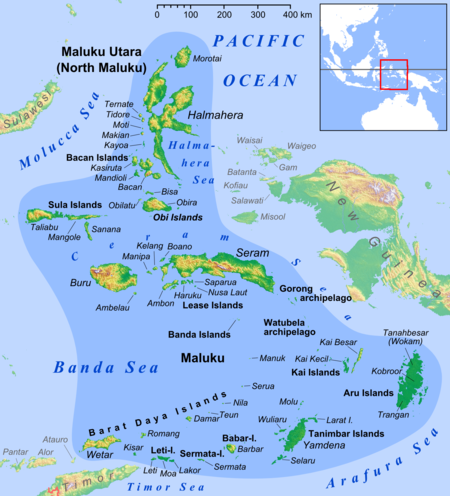
One of several small seas in Indonesia Seram SeaLaut Seram (Indonesian)Location of the Ceram Sea within Southeast Asia.Seram SeaCoordinates2°20′S 128°00′E / 2.333°S 128.000°E / -2.333; 128.000TypeSeaBasin countriesIndonesiaSurface area120,000 km2 (46,000 sq mi) Ceram Sea in the center of Maluku Islands The Seram Sea or Ceram Sea (Indonesian: Laut Seram) is one of several small seas between the scattered islands of Indonesia. It is a sec...

Artikel ini membutuhkan rujukan tambahan agar kualitasnya dapat dipastikan. Mohon bantu kami mengembangkan artikel ini dengan cara menambahkan rujukan ke sumber tepercaya. Pernyataan tak bersumber bisa saja dipertentangkan dan dihapus.Cari sumber: Daftar acara GTV – berita · surat kabar · buku · cendekiawan · JSTOR Logo GTV Halaman ini memuat daftar acara GTV. Program saat ini Film Big Movies CandyLand Family Lolipop Platinum Platinum Blockbuster (dihe...

American politician (1815–1903) Senator Ramsey redirects here. For other uses, see Senator Ramsey (disambiguation). For the English footballer, see Alexander Ramsey (footballer). For people with a similar name, see Alexander Ramsay. Alexander RamseyChairman Utah CommissionIn officeMarch 1882 – 1885Succeeded byAmbrose B. Carlton34th United States Secretary of WarIn officeDecember 10, 1879 – March 5, 1881Preceded byGeorge W. McCrarySucceeded byRobert LincolnUnited States...

52nd Infantry Division Torino52nd Infantry Division Torino insigniaActive1940–1943Country Kingdom of ItalyBranch Royal Italian ArmyRoleInfantrySizeDivisionEngagementsWorld War IICommandersNotablecommandersUgo de CarolisInsigniaIdentificationsymbol Torino Division gorget patchesMilitary unit The 52nd Infantry Division Torino (Italian: 52ª Divisione di fanteria Torino) was an infantry division of the Royal Italian Army during World War II. The Torino was named after the city of Tu...

У этого термина существуют и другие значения, см. Бомбарда (значения). Поплавок-бомбарда Бомба́рда (техника ловли) или Сбирули́но (техника ловли) — представляет собой объединение спиннингового и нахлыстового видов ловли рыбы. В ней используется удлинённое подобие спи�...

Election 1928 Massachusetts gubernatorial election ← 1926 November 6, 1928 (1928-11-06) 1930 → Nominee Frank G. Allen Charles H. Cole Party Republican Democratic Popular vote 769,372 750,137 Percentage 50.06% 48.81% County resultsAllen: 40–50% 50–60% 60–70% 70–80%Cole: 60–70% Governor be...

Patrick McHenry Patrick Timothy McHenry (lahir 22 Oktober 1975) adalah anggota DPR Amerika Serikat. Ia adalah anggota Partai Republik. Ia sebelumnya juga menjabat sebagai anggota DPRD Carolina Utara untuk masa jabatan tunggal. Pranala luar Congressman Patrick McHenry official U.S. House website Patrick McHenry for Congress Patrick McHenry di Curlie (dari DMOZ) Kemunculan di C-SPAN Biografi di Biographical Directory of the United States Congress Catatan suara dikelola oleh The Washington Post ...

PT Medco Energi Internasional TbkThe Energy Tower, kantor pusat perusahaan iniNama dagangMedcoEnergiSebelumnyaPT Meta Epsi Pribumi Drilling Company (1980 - 1994)JenisPerusahaan publikKode emitenIDX: MEDCIndustriEnergiDidirikan9 Juni 1980; 43 tahun lalu (1980-06-09)PendiriArifin PanigoroKantorpusatJakarta, IndonesiaWilayah operasiIndonesiaTokohkunciHilmi Panigoro[1](Direktur Utama)Yani Panigoro[2](Komisaris Utama)ProdukMinyak bumiGas alamListrikTembagaEmasPendapatan US$ 2,...

شعار مكافحة التنمر قانون مكافحة التنمر هو تشريع تم سنه للمساعدة في الحد من ثقافة التنمر والقضاء عليها. قد يكون هذا التشريع وطنيًا أو دوليًا ويهدف عادة إلى إنهاء التنمر في المدارس أو أماكن العمل. الولايات المتحدة أقرت جميع الولايات الخمسين في الولايات المتحدة تشريعًا لمكاف...

العلاقات الصينية الكينية الصين كينيا الصين كينيا تعديل مصدري - تعديل العلاقات الصينية الكينية هي العلاقات الثنائية التي تجمع بين الصين وكينيا.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين: وجه المقارنة الصين كينيا ال�...

1933 document signed by German academics Title page of the document Bekenntnis der Professoren an den Universitäten und Hochschulen zu Adolf Hitler und dem nationalsozialistischen Staat officially translated into English as the Vow of allegiance of the Professors of the German Universities and High-Schools to Adolf Hitler and the National Socialistic State was a document presented on 11 November 1933 at the Albert Hall in Leipzig. It had statements in German, English, Italian, and Spanish by...

Questa voce o sezione sull'argomento fiumi non cita le fonti necessarie o quelle presenti sono insufficienti. Puoi migliorare questa voce aggiungendo citazioni da fonti attendibili secondo le linee guida sull'uso delle fonti. Segui i suggerimenti del progetto di riferimento. EufrateIl fiume Eufrate presso Abu KamalStati Turchia Siria Iraq Lunghezza2 760 km Portata media818 m³/s Bacino idrografico765 831 km² Altitudine sorgente4 500 m s.l.m. NasceTurchia S...

Bahasa Jepang Pertengahan Akhir 中世日本語 WilayahJepangEraberkembang menjadi bahasa Jepang Modern Awal pada abad ke-17 Rumpun bahasaJaponik JepangJepang Pertengahan Akhir Bentuk awalJepang Kuno Jepang Pertengahan AwalJepang Pertengahan Akhir Sistem penulisanHiraganaKatakanaAksara HanKode bahasaISO 639-3–GlottologTidak ada Status pemertahanan Punah EXSingkatan dari Extinct (Punah)Terancam CRSingkatan dari Critically endangered (Terancam Kritis) SESingkatan dari Severely endangered (Ter...

Este artículo o sección necesita referencias que aparezcan en una publicación acreditada. Busca fuentes: «Archiducado de Austria» – noticias · libros · académico · imágenesEste aviso fue puesto el 3 de agosto de 2013. Archiducado de AustriaErzherzogtum Österreich Estado imperial y tierra de la corona 1453-1804Bandera(1359-1804)Escudo(1359-1804) Las tierras de los Habsburgo en 1635; en rojo el archiducado de Austria, en azul las tierras de la Corona de Bohemia,...

Сельское поселениеСельское поселение Михайло-Овсянка Страна Россия Входит в Пестравский район Включает 1 населённый пункт Адм. центр село Михайло-Овсянка Глава Абрамов Иван Анатольевич История и география Часовой пояс MSK (UTC+3) Население Население ↘599[1] чел. ...
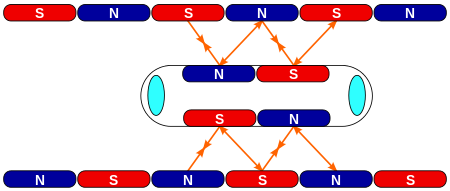
Запрос «ТП-01» перенаправляется сюда. На эту тему нужно создать отдельную статью. Поезд на магнитной подвеске в Шанхае, Китай Поезд на магнитной подушке, магнитопла́н или маглев (от англ. magnetic levitation «магнитная левитация») — транспортный термин — поезд и трамвай, у�...

La contea di Chalon del Ducato di Borgogna si trova in Saona e Loira, nella regione della Borgogna del Medioevo. Indice 1 Conti sotto i Carolingi 2 Contea divisa 2.1 Conti di metà contea 2.2 Conti dell'altra metà contea 3 Contea riunificata 4 Fine della Contea di Chalon 5 Note 6 Bibliografia 7 Voci correlate 8 Collegamenti esterni Conti sotto i Carolingi Stemma della Borgogna Questa è la lista dei conti Chalon: Adalardo di Chalon († 763), conte di Chalon, menzionato dal 763 al 765[1&...