Boronic acid
|
Read other articles:

Elang jawa Nisaetus bartelsi Status konservasiGentingIUCN22696165 TaksonomiKerajaanAnimaliaFilumChordataKelasAvesOrdoAccipitriformesFamiliAccipitridaeGenusNisaetusSpesiesNisaetus bartelsi (Stresem., 1924) Tata namaSinonim taksonJavan hawk-eagle (en) ProtonimSpizaetus nipalensis bartelsi DistribusiEndemikJawa lbs Elang jawa (Nisaetus bartelsi) adalah salah satu spesies elang berukuran sedang dari keluarga Accipitridae dan genus Nisaetus yang endemik di Pulau Jawa. Satwa ini dianggap identik de...

AgordatUna fotografia dell'AgordatDescrizione generale Tipoincrociatore torpediniere (1900-1914)esploratore (1914-1921)cannoniera (1921-1923) ClasseAgordat Proprietà Regia Marina CostruttoriRegio Arsenale, Castellammare di Stabia Impostazione18 febbraio 1897 Varo11 ottobre 1899 Entrata in servizio26 settembre 1900 Radiazione4 gennaio 1923 Destino finaledemolito Caratteristiche generaliDislocamentocarico normale 1340 tpieno carico 1530 t Lunghezza91,6 m Larghezza9,3 m Pescaggio4...

Seorang bapak rumah tangga sedang mengasuh anak-anak Bapak rumah tangga adalah ayah yang tidak bekerja tetapi melakukan berbagai pekerjaan rumah tangga, seperti memasak, bersih-bersih, serta merawat dan membesarkan anak di rumah. Jumlah bapak rumah tangga mulai meningkat secara bertahap pada akhir abad ke-20, terutama di negara-negara Barat yang maju. Statistik terbaru yang dirilis Pew Research, menunjukkan sebuah laporan pada Juni 2014 yang menemukan 2 juta pria menjadi bapak rumah tangga di...

2nd-3rd century Greek peripatetic philosopher Alexander of Aphrodisias16th century CE engravingBorn2nd century ADAphrodisias, Caria(modern-day Geyre, Karacasu, Aydın, Turkey)Died3rd century ADAthensSchoolPeripateticsAlexandrists (posthumously) Opening paragraph of the treatise On Fate (Peri eimarmenes) by Alexander of Aphrodisias dedicated to Emperors (autokratoras). From an anonymous edition published in 1658. Alexander of Aphrodisias (Greek: Ἀλέξανδρος ὁ Ἀφροδισιεύ�...

أريثوسا خريطة الموقع تقسيم إداري البلد اليونان [1] خصائص جغرافية إحداثيات 40°44′36″N 23°35′18″E / 40.743333333333°N 23.588333333333°E / 40.743333333333; 23.588333333333 الارتفاع 374 متر السكان التعداد السكاني 564 (resident population of Greece) (2021)813 (resident population of Greece) (2001)858 (resident population of Greece) (1991)7...

Kugsak-45 adalah klub olahraga dari Greenland yang berbasis di Qasigiannguit. Mereka bersaing di Coca Cola GM. Kugsak-45Nama lengkapKugsak-45Berdiri1945StadionStadion QasigiannguitQasigiannguit, Greenland(Kapasitas: Tidak diketahui)KetuaKristian RosingLigaCoca Cola GM20087th Kostum kandang Kostum tandang Prestasi Coca Cola GM: 2 Juara: 1995, 2002 Pranala luar Situs resmi Asosiasi Sepak Bola Greenland Situs web Remotest Football[pranala nonaktif]

Radio FGWilayah siarPrancisFrekuensiBermacam sesuai kotaMulai mengudara1981PemilikFG ConceptSitus webwww.radiofg.com Radio FG (sebelumnya FG DJ Radio) merupakan sebuah stasiun radio berbahasa Prancis yang mulai mengudara dari Paris, Prancis dalam 98.2 FM. Merupakan stasiun radio yang menyiarkan musik House, Techno, Dance dan R'n'B. Sejarah Radio FG didirikan tahun 1981 sebagai radio komunitas yang berakar pada adegan homoseksual Paris dan mendukung komunitas homoseksual. 'FG' dikatakan memili...

Subregion of the Asian continent For other uses, see East Asia (disambiguation). East AsiaArea11,840,000 km2 (4,570,000 sq mi) (3rd)Population1.6 billion (2023; 4th)Population density141.9 km2 (54.8 sq mi)GDP (PPP)$44.7 trillion (2023)[1]GDP (nominal)$24.8 trillion (2023)[1]GDP per capita$15,000 (nominal)[1]DemonymEast AsianCountries 6 countries[2][3][4][5] China Japan Mongolia Nort...

PrestoType of sitePrivateAvailable inEnglishFounded13 March 2014; 10 years ago (2014-03-13)Dissolved31 January 2017; 7 years ago (2017-01-31)Successor(s)Foxtel NowHeadquartersSydney, New South Wales, AustraliaArea servedAustraliaOwnerFoxtelServicesstreaming serviceRegistrationMonthly subscription required to access contentLaunched15 January 2015; 9 years ago (2015-01-15)Current statusDefunct Presto was an Australian media s...
2020年夏季奥林匹克运动会波兰代表團波兰国旗IOC編碼POLNOC波蘭奧林匹克委員會網站olimpijski.pl(英文)(波兰文)2020年夏季奥林匹克运动会(東京)2021年7月23日至8月8日(受2019冠状病毒病疫情影响推迟,但仍保留原定名称)運動員206參賽項目24个大项旗手开幕式:帕维尔·科热尼奥夫斯基(游泳)和马娅·沃什乔夫斯卡(自行车)[1]闭幕式:卡罗利娜·纳亚(皮划艇)&#...

خط زمني للفروع الأساسية للكنائس المسيحية بحسب العقيدة. تاريخ المسيحية، ويعنى بهذا دراسة تاريخ الديانة المسيحية والكنيسة، منذ يسوع ورسله الإثني عشر حتى أيامنا الحاضرة. والديانة المسيحية هي ديانةٌ توحيدية أقيمت على أساس تعاليم وحياة يسوع. أما الكنيسة بمعناها اللاهوتي والم...

US Navy pilot and first American casualty of the Persian Gulf War Michael Scott SpeicherSpeicher in 1990Born(1957-07-12)July 12, 1957Kansas City, Missouri, U.S.DiedJanuary 17, 1991(1991-01-17) (aged 33)Tulul ad Dulaym, Al Anbar Governorate, IraqBuriedJacksonville Memory Gardens, Orange Park, Florida, U.S.Allegiance United StatesService/branch United States NavyRank Captain (posthumous)Unit VFA-81 SunlinersBattles/warsPersian Gulf War Operation Desert Storm † AwardsPu...

artikel ini tidak memiliki pranala ke artikel lain. Tidak ada alasan yang diberikan. Bantu kami untuk mengembangkannya dengan memberikan pranala ke artikel lain secukupnya. (Pelajari cara dan kapan saatnya untuk menghapus pesan templat ini) Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Desember 2022. artikel in...

Light rail station 17th St/SMC 17th Street/SMC station platform in 2016General informationLocation1701 Colorado AvenueSanta Monica, CaliforniaCoordinates34°01′25″N 118°28′47″W / 34.0237°N 118.4797°W / 34.0237; -118.4797Owned byLos Angeles County Metropolitan Transportation AuthorityPlatforms1 island platformTracks2ConnectionsBig Blue BusConstructionParking70 spaces[1]Bicycle facilitiesMetro Bike Share station,[2] racks and lockers[3...

English rugby union football club For other uses of London Scottish, see London Scottish (disambiguation). Rugby teamLondon ScottishFull nameLondon Scottish Football ClubUnionMiddlesex RFU, Scottish RUNickname(s)The Exiles, ScottishFounded1878; 146 years ago (1878)LocationRichmond, London, EnglandGround(s)Richmond Athletic Ground (Capacity: 4,500 (1,000 seated))PresidentPaul BurnellDirector of RugbyBryan RedpathCoach(es)Joe GrayCaptain(s)Joe ReesLeague(s)RFU Championship2022...

Questa voce sull'argomento stagioni delle società calcistiche italiane è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Voce principale: Unione Sportiva Aosta. Associazione Sportiva AostaStagione 1942-1943Sport calcio Squadra Aosta Allenatore Mario Malatesta Presidente Raffaele Tognoni Serie C9º posto nel girone E. 1941-1942 1945-1946 Si invita a seguire il modello di voce Questa voce raccoglie l...

Dambel komune di Italia Dambel (it) Tempat NegaraItaliaDaerah otonom dengan status istimewaTrentino-Tirol SelatanProvinsi di ItaliaTrentino NegaraItalia Ibu kotaDambel PendudukTotal412 (2023 )GeografiLuas wilayah5,15 km² [convert: unit tak dikenal]Ketinggian750 m Berbatasan denganNovella (en) Romeno Sarnonico Sanzeno Informasi tambahanKode pos38010 Zona waktuUTC+1 UTC+2 Kode telepon0463 ID ISTAT022071 Kode kadaster ItaliaD246 Lain-lainSitus webLaman resmi Peta wilayah komune Dambe...

Mengusir setan dari seorang anak laki-laki yang kerasukan setan dari Très Riches Heures du Duc de Berry, abad ke-15. Pengusiran roh dari seorang anak yang kerasukan setan, atau anak laki-laki dengan roh bisu,[1] adalah salah satu dari mukjizat-mukjizat yang dikaitkan dengan Yesus dan yang dilaporkan dalam ketiga Injil Sinoptik, yang melibatkan penyembuhan penyakit yang dimiliki anak itu melalui pengusiran setan. Catatan dalam Injil Markus agak berbeda dengan catatan dalam Injil Matiu...

Challenger Lugano 2008Sport Tennis Data30 giugno - 6 luglio Edizione10ª CampioniSingolare Luis Horna Doppio Rameez Junaid / Philipp Marx 2007 2009 Il Challenger Lugano 2008 è stata la decima edizione di un torneo di tennis facente parte della categoria ATP Challenger Series nell'ambito dell'ATP Challenger Series 2008. Il torneo si è giocato a Lugano in Svizzera dal 30 giugno al 6 luglio 2008 su campi in terra rossa e aveva un montepremi di €85 000+H. Indice 1 Vincitori 1.1 Singolare 1.2 ...

State of completeness, flawlessness, or supreme excellence For other uses, see Perfection (disambiguation). Perfection is a state, variously, of completeness, flawlessness, or supreme excellence. The term is used to designate a range of diverse, if often kindred, concepts. These have historically been addressed in a number of discrete disciplines, notably mathematics, physics, chemistry, ethics, aesthetics, ontology, and theology.[1] Term and concept Aristotle The form of the word lon...




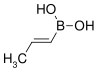




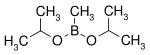
![The Suzuki reaction {\displaystyle {\begin{matrix}{}\\{\ce {{R1-BY2}+R2-X->[{\underset {\text{catalyst}}{\text{Pd}}}][{\text{Base}}]R1-R2}}\\{}\end{matrix}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c35df36f38fec8abbc8e9d1d9f04e9b2687ae245)










