|
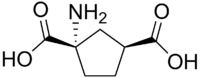
1-Amino-1,3-dikarboksiciklopentan
| 1-Amino-1,3-dikarboksiciklopentan
|
|
| Naziv po klasifikaciji
|
1-Aminociklopentan-1,3-dikarboksilna kiselina[1]
|
| Identifikacija
|
| Abrevijacija
|
ACPD
|
| CAS registarski broj
|
39026-63-6  Y, 56827-69-1 (1R,3S), 111900-32-4 (1S,3R) Y, 56827-69-1 (1R,3S), 111900-32-4 (1S,3R)
|
| PubChem[2][3]
|
1310,
44381972 (1S),
231345 (1R,3R),
73537 (1R,3S),
104766 (1S,3R),
6604704 (1S,3S)
|
| ChemSpider[4]
|
1270  Y, 201559 (1R,3R) Y, 201559 (1R,3R)  Y, 66220 (1R,3S) Y, 66220 (1R,3S)  Y, 94574 (1S,3R) Y, 94574 (1S,3R)  Y, 5036967 (1S,3S) Y, 5036967 (1S,3S)  Y Y
|
| MeSH
|
1-amino-1,3-dicarboxycyclopentane
|
| ChEMBL[5]
|
CHEMBL168278  Y Y
|
| RTECS registarski broj toksičnosti
|
GY4060000 (1S,3R)
|
| Jmol-3D slike
|
Slika 1
|
|
|
|
|---|
InChI=1S/C7H11NO4/c8-7(6(11)12)2-1-4(3-7)5(9)10/h4H,1-3,8H2,(H,9,10)(H,11,12)  Y Y
Kod: YFYNOWXBIBKGHB-UHFFFAOYSA-N  Y Y |
|
| Svojstva
|
| Molekulska formula
|
C7H11NO4
|
| Molarna masa
|
173.17 g mol−1
|
| Agregatno stanje
|
Beli kristali
|
| Rastvorljivost u vodi
|
20 g dm-3
|
| Rastvorljivost u etanol
|
240 mg dm-3
|
| log P
|
-0,709
|
| pKa
|
2,112
|
| Baznost (pKb)
|
11,885
|
| Izoelektrična tačka
|
2,84
|
| Opasnost
|
| EU-klasifikacija
|
 Xn Xn
|
| R-oznake
|
R20/21/22, R36/37/38
|
| S-oznake
|
S26, S36/37
|
|
 Y (šta je ovo?)
(verifikuj) Y (šta je ovo?)
(verifikuj)
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala
|
| Infobox references
|
1-Amino-1,3-dikarboksiciklopentan (ACPD) je hemijsko jedinjenje koje se vezuje za metabotropni glutamatni receptor (mGluR).[6] ACPD deluje kao mGluR agonist. On je krut analog neurotransmitera glutamata koji ne aktivira jonotropne glutamatne receptore.[7] ACPD može da indukuje konvulzije kod neonatalnih pacova.[8]
Reference
- ↑ „1-amino-1,3-dicarboxycyclopentane - Compound Summary”. PubChem Compound. USA: National Center for Biotechnology Information. 25. 3. 2005.. Identification and Related Records. Pristupljeno 15. 10. 2011.
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846. edit
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594. edit
- ↑ Schoepp DD, True RA (September 1992). „1S,3R-ACPD-sensitive (metabotropic) [3H]glutamate receptor binding in membranes”. Neurosci. Lett. 145 (1): 100–4. DOI:10.1016/0304-3940(92)90213-Q. PMID 1461560.
- ↑ Manzoni O, Fagni L, Pin JP, Rassendren F, Poulat F, Sladeczek F, Bockaert J (July 1990). „(trans)-1-amino-cyclopentyl-1,3-dicarboxylate stimulates quisqualate phosphoinositide-coupled receptors but not ionotropic glutamate receptors in striatal neurons and Xenopus oocytes”. Mol. Pharmacol. 38 (1): 1–6. PMID 2164627. [mrtav link]
- ↑ McDonald JW, Fix AS, Tizzano JP, Schoepp DD (October 1993). „Seizures and brain injury in neonatal rats induced by 1S,3R-ACPD, a metabotropic glutamate receptor agonist”. J. Neurosci. 13 (10): 4445–55. PMID 8410197.
|
|---|
| Jonotropni | | |
|---|
| Agonisti: Konkurentni agonisti: Aspartat • Glutamat • Homokinolinska kiselina • Ibotenska kiselina • NMDA • Kinolinska kiselina • Tetrazolilglicin; Agonisti glicinskog mesta: ACBD • ACPC • ACPD • Alanin • CCG • Cikloserin • DHPG • Fluoroalanin • Glicin • HA-966 • L-687,414 • Milacemid • Sarkozin • Serin • Tetrazolilglicin; Agonisti poliaminskog mesta: Akamprosat • Spermidin • SperminAntagonisti: Konkurentni antagonisti: AP5 (APV) • AP7 • CGP-37849 • CGP-39551 • CGP-39653 • CGP-40116 • CGS-19755 • CPP • LY-233,053 • LY-235,959 • LY-274,614 • MDL-100,453 • Midafotel (d-CPPen) • NPC-12,626 • NPC-17,742 • PBPD • PEAQX • Perzinfotel • PPDA • SDZ-220581 • Selfotel; Nekonkurentni antagonisti: ARR-15,896 • Karoverin • Deksanabinol • FPL-12495 • FR-115,427 • Hodgkinsin • Magnezijum • MDL-27,266 • NPS-1506 • Psihotridin • Cink; Nekonkurentni blokatori pora: 2-MDP • 3-MeO-PCP • 8A-PDHQ • Amantadin • Aptiganel • ARL-12,495 • ARL-15,896-AR • ARL-16,247 • Budipin • Delucemin • Deksoksadrol • Dekstralorfan • Dieticiklidin • Dizocilpin • Endopsihozin • Esketamin • Etoksadrol • Eticiklidin • Gaciklidin • Ibogain • Indantadol • Ketamin • Ketobemidon • Loperamid • Memantin • Meperidin (Petidin) • Metadon • Metorfan ( Dekstrometorfan, Levometorfan) • Metoksetamin • Milnacipran • Morfanol ( Dekstrorfan, Levorfanol) • NEFA • Nerameksan • Azotsuboksid • Noribogain • Orfenadrin • PCPr • Fenciklamin • Fenciklidin • Propoksifen • Remacemid • Rinhofilin • Riluzol • Rimantadin • Roliciklidin • Sabeluzol • Tenociklidin • Tiletamin • Tramadol • Ksenon; Antagonisti glicinskog mesta: ACEA-1021 • ACEA-1328 • ACC • Karisoprodol • CGP-39653 • CKA • DCKA • Felbamat • Gavestinel • GV-196,771 • Kinurenska kiselina • L-689,560 • L-701,324 • Lakosamid • Likostinel • LU-73,068 • MDL-105,519 • Meprobamat • MRZ 2/576 • PNQX • ZD-9379; Antagonisti NR2B podjedinice: Bezonprodil • CO-101,244 (PD-174,494) • CP-101,606 • Eliprodil • Haloperidol • Ifenprodil • Izoksuprin • Nilidrin • Ro8-4304 • Ro25-6981 • Traksoprodil; Neklasifikovani/nesortirani antagonisti: Hloroform • Dietil etar • Enfluran • Etanol (Alkohol) • Halotan • Izofluran • Metoksifluran • Toluen • Trihloroetan • Trihloroetanol • Trihloroetilen • Ksilen |
|---|
| |
|---|
|
|---|
| Metabotropni | |
|---|
Inhibitori
transporta | |
|---|
|
|