Uranium(III) hydride
| |||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Untuk the remotely controlled vehicle used in bomb disposal, lihat Wheelbarrow (EOD). Untuk the aviation risk, lihat Wheel-barrowing. Gerobak tangan. Gerobak tangan yang lebih kuno Gerobak tangan atau kereta sorong adalah alat kecil untuk membawa barang yang biasanya mempunyai satu roda saja. Gerobak didesain untuk didorong dan dikendalikan oleh seseorang menggunakan dua pegangan di bagian belakang gerobak. Pada masa lalu Gerobak juga dibantu dorongan angin yang ditangkap oleh sebuah layar ya...

Bagian dari seriIslam Rukun Iman Keesaan Allah Malaikat Kitab-kitab Allah Nabi dan Rasul Allah Hari Kiamat Qada dan Qadar Rukun Islam Syahadat Salat Zakat Puasa Haji Sumber hukum Islam al-Qur'an Sunnah (Hadis, Sirah) Tafsir Akidah Fikih Syariat Sejarah Garis waktu Muhammad Ahlulbait Sahabat Nabi Khulafaur Rasyidin Khalifah Imamah Ilmu pengetahuan Islam abad pertengahan Penyebaran Islam Penerus Muhammad Budaya dan masyarakat Akademik Akhlak Anak-anak Dakwah Demografi Ekonomi Feminisme Filsafat...

Census-designated place in Maryland, United StatesPotomac, MarylandCensus-designated placeGreat Falls TavernLocation of Potomac in MarylandCoordinates: 39°00′12″N 77°12′20″W / 39.00333°N 77.20556°W / 39.00333; -77.20556Country United StatesState MarylandCounty MontgomeryFirst settled1714; 310 years ago (1714)Area[1] • Total26.58 sq mi (68.85 km2) • Land25.14 sq mi (65.12&#...

Haus am Checkpoint Charlie Monumen Kebebasan Museum Titik Pengecekan Charlie (Jerman: Haus am Checkpoint Charliecode: de is deprecated atau Mauermuseum) adalah sebuah museum di Berlin. Museum tersebut mengambil nama dari titik perlintasan terkenal di Tembok Berlin, dan dibuat untuk mendokumentasikan apa yang disebut sistem keamanan perbatasan terbaik di dunia (dalam kata-kata jenderal Jerman Timur Heinz Hoffmann). Simpanannya adalah foto-foto dan dokumen-dokumen terkait dari upaya pelarian su...

追晉陸軍二級上將趙家驤將軍个人资料出生1910年 大清河南省衛輝府汲縣逝世1958年8月23日(1958歲—08—23)(47—48歲) † 中華民國福建省金門縣国籍 中華民國政党 中國國民黨获奖 青天白日勳章(追贈)军事背景效忠 中華民國服役 國民革命軍 中華民國陸軍服役时间1924年-1958年军衔 二級上將 (追晉)部队四十七師指挥東北剿匪總司令部參謀長陸軍�...

Elisabetta di Borbone-FranciaElisabetta di Francia ritratta da Elisabeth Vigée-Lebrun nel 1782, Reggia di VersaillesPrincipessa di FranciaStemma Nome completoÉlisabeth Philippe Marie Hélène de France TrattamentoSua Altezza Reale Altri titoliFille de France NascitaReggia di Versailles, 3 maggio 1764 MortePlace de la Concorde, Parigi, 10 maggio 1794 Luogo di sepolturaCimitero degli Errancis, Parigi; in seguito alle Catacombe di Parigi (forse) PadreLuigi Ferdinando, delfino di Francia ...

WrestleMania 38Poster prmosi menampilkan Brock Lesnar and Roman ReignsTaglineThe Most Stupendous Two-Night Event In WrestleMania HistoryInformasiPromotorWWEMerek Raw SmackDown TanggalApril 2–3, 2022Kehadiran Malam 1: 77,899[1][2] Malam 2: 78,453[3][4] Dikombinasikan: 156,352 (diperdebatkan)[5] TempatAT&T StadiumLokasiArlington, TexasKronologi acara WWE Network NXT Stand & Deliver WrestleMania 38 WrestleMania Backlash Kronologi WrestleMania 37 ...

Северный морской котик Самец Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:Синапси...

穆罕默德·达乌德汗سردار محمد داود خان 阿富汗共和國第1任總統任期1973年7月17日—1978年4月28日前任穆罕默德·查希爾·沙阿(阿富汗國王)继任穆罕默德·塔拉基(阿富汗民主共和國革命委員會主席團主席) 阿富汗王國首相任期1953年9月7日—1963年3月10日君主穆罕默德·查希爾·沙阿 个人资料出生(1909-07-18)1909年7月18日 阿富汗王國喀布尔逝世1978年4月28日(...

مديرية حرف سفيان - مديرية - تقسيم إداري البلد اليمن[1] العاصمة الحرف، عمران[2] المحافظة محافظة عمران خصائص جغرافية إحداثيات 16°35′00″N 44°00′00″E / 16.58333°N 44°E / 16.58333; 44 المساحة 2733.90 كم² الارتفاع 2076 متر السكان الكثافة السكانية 155.3 نسمة/كم2 ا�...

Suasana di Harajuku Harajuku (原宿code: ja is deprecated ) adalah sebutan populer untuk kawasan di sekitar Stasiun JR Harajuku, Distrik Shibuya, Tokyo. Kawasan ini terkenal sebagai tempat anak-anak muda berkumpul dengan menggunakan pakaian yang nyentrik dan mencolok. Lokasinya mencakup sekitar Kuil Meiji, Taman Yoyogi, pusat perbelanjaan Jalan Takeshita (Takeshita-dōri), department store Laforet, dan Gimnasium Nasional Yoyogi. Harajuku bukan sebutan resmi untuk nama tempat, dan tidak dican...

British hatchback from Mini, marque of BMW For the 1959–2000 Mini models, see Mini. Motor vehicle MiniMini One (F56)OverviewManufacturerBMWAlso calledMini Cooper (2001–present)Mini John Cooper Works (2008–present)Mini Hatch (2001–present)Mini Hardtop (2001–present)Mini One (2001–2022)[1]Production2001–presentModel years2002–presentBody and chassisClassSupermini (B)Sport compact / hot hatch (Cooper S & JCW)LayoutFront-engine, front-wheel-driveChronologyPre...

Chemical operations that separate fissile material from spent fuel to be recycled as new fuel This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (May 2012) (Learn how and when to remove this message) Sellafield nuclear reprocessing site, UK Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel.[1] Origin...

فالسيربيرخ: نقطة حدودية ثلاثية بين (ألمانيا/هولندا/بلجيكا) النقطة الحدودية الثلاثية هي عبارة عن موقع جغرافي يمثل نقطة التقاء الحدود السياسية لثلاثة بلدان.[1][2][3] يمكن أن تكون النقطة برية، وعنذئذ غالباً ما تعلم بنصب، أو أن تكون مائية (نهر أو بحيرة أو بحر). من المنط...

此條目需要补充更多来源。 (2023年11月11日)请协助補充多方面可靠来源以改善这篇条目,无法查证的内容可能會因為异议提出而被移除。致使用者:请搜索一下条目的标题(来源搜索:伊壁鸠鲁 — 网页、新闻、书籍、学术、图像),以检查网络上是否存在该主题的更多可靠来源(判定指引)。 伊壁鸠鲁ἘπίκουροςEpicurus出生前341年薩摩斯島逝世前270年馬其頓王國雅典�...

Era of Ancient Egyptian historyFirst Intermediate Period of Egyptc. 2181 BC–c. 2055 BCCapital Memphis (c. 2181 BC – c. 2160 BC), Seventh Dynasty–Eighth Dynasty Heracleopolis Magna (c. 2160 BC – c. 2050 BC), Ninth Dynasty–Tenth Dynasty Thebes (c. 2134 BC – c. 2061 BC), Eleventh Dynasty of Egypt Common languagesAncient EgyptianReligion Ancient Egyptian religionGovernmentMonarchyPharaoh • c. ...
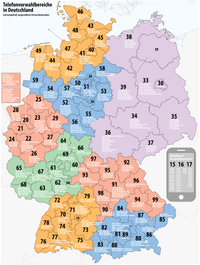
Telephone numbers in Germany8 geographic zonesLocationCountryGermanyContinentEuropeRegulatorFederal Network AgencyTypeOpenNSN length3 to 13[1]Format(xx…) xx…Access codesCountry code49International access00Long-distance0List of Germany dialing codes The regulation of telephone numbers in Germany is the responsibility of the Federal Network Agency (German: Bundesnetzagentur, BNetzA) of the German government. The agency has a mandate to telecommunications in Germany and other infras...

Die General Assembly tagt im North Carolina State Legislative Building Die North Carolina General Assembly ist die State Legislature und damit die Legislative des US-Bundesstaats North Carolina und wurde durch die staatliche Verfassung 1776 geschaffen. Sie besteht aus dem Repräsentantenhaus von North Carolina, das als Unterhaus fungiert, sowie dem Senat von North Carolina als Oberhaus. Die General Assembly tagt im North Carolina State Legislative Building in Raleigh. Das Repräsentantenhaus ...

Political entities in the Indian subcontinent from 3rd century BCE - 13th century CE This is a dynamic list and may never be able to satisfy particular standards for completeness. You can help by adding missing items with reliable sources. This article is written like a personal reflection, personal essay, or argumentative essay that states a Wikipedia editor's personal feelings or presents an original argument about a topic. Please help improve it by rewriting it in an encyclopedic style. (J...

Special shape of attic amphoras Athena on a Panathenic amphora (National Archaeological Museum of Athens) Panathenaic amphorae were the amphorae, large ceramic vessels, that contained the olive oil given as a prize in the Panathenaic Games. Some were ten imperial gallons (12 US gal; 45 L) and 60–70 cm (24–28 in) high. This oil came from the sacred grove of Athena at Akademia. The amphorae which held it had the distinctive form of tight handles, narrow neck and fee...