Opicapone
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Santo CataldPatung Santo Catald di Taranto.Lahirabad ke-7[1]IrlandiaMeninggal685TarantoDihormati diTarento, IrlandiaKanonisasiSekitar 685 (Pre-jemaat)Tempat ziarahLismore, Provinsi Waterford, TarantoPesta10 MeiPelindungDipuja untuk melindungi dari wabah penyakit, musibah kekeringan dan badai Santo Catald dari Taranto (aka Cataldus, Cathaluds, Cathaldus, Cat(t)aldo, Cathal) merupakan seorang biarawan Irlandia yang berasal dari abad ke-7.[2] Biografi Biaranya berada di Lismore, ...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Oktober 2022. Buku ilmu antik adalah karya sejarah asli, misalnya buku atau makalah teknis, tentang sains, matematika, dan kadang-kadang teknik. Buku-buku ini adalah rujukan utama yang penting untuk kajian sejarah sains dan teknologi, yang dapat memberikan wawasan b...

Bajaj adalah grup lawak Indonesia yang beranggotakan Aden Bajaj, Isa Bajaj, dan Melky Bajaj. Grup lawak ini memulai karier dengan mengikuti audisi pencarian pelawak yang dibuat oleh TPI. Kemudian Bajaj semakin dikenal luas publik dengan bermain dalam sinetron Para Pencari Tuhan, sinetron Bajaj Gaul di Global TV bersama dengan George Taka, dan acara lawak Cagur Naik Bajaj di ANTV bersama dengan grup lawak Cagur. Filmografi Bebek Belur (2010) Penganten Pocong (2012) Nenek Gayung (2012) Sinetron...
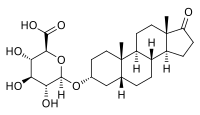
Etiocholanolone glucuronide Names IUPAC name 17-Oxo-5β-androstan-3α-yl β-D-glucopyranosiduronic acid Systematic IUPAC name (2S,3S,4S,5R,6R)-6-{[(3aS,3bR,5aR,7R,9aS,9bS,11aS)-9a,11a-Dimethyl-1-oxohexadecahydro-1H-cyclopenta[a]phenanthren-7-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid Other names 5β-Androstan-3α-ol-17-one 3-glucuronide; 3α-Hydroxy-5β-androstan-17-one 3-glucuronide; Etiocholan-3α-ol-17-one 3-glucuronide; 3α-Hydroxyetiocholan-17-one 3-glucuronide; 17-oxoetiocholan-3�...

American basketball player (born 1963) This biography of a living person needs additional citations for verification. Please help by adding reliable sources. Contentious material about living persons that is unsourced or poorly sourced must be removed immediately from the article and its talk page, especially if potentially libelous.Find sources: Adrian Branch – news · newspapers · books · scholar · JSTOR (October 2023) (Learn how and when to remove th...
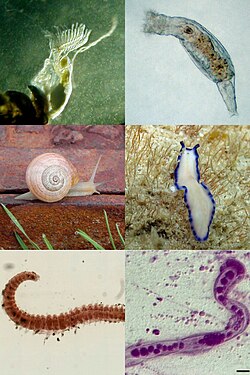
Spiralia Periode Kriogenium — TaksonomiKerajaanAnimaliaSuperfilumSpiralia Waldemar Schleip, 1929 Klad Gnathifera Platytrochozoa lbs Spiralia adalah sebuah klad dari hewan protostomia, termasuk moluska, annelida, platyhelminthes dan masih banyak lagi.[1] Istilah Spiralia dipakai untuk mengelompokkan hewan yang melakukan pembelahan spiral saat menjadi embrio.[2] Distribusi spiralia lintas filogeni Spesies dari moluska, annelida, platyhelminthes dan nemertea menunjukkan pembela...

Stereoisomerism due to hindered rotation Atropisomers of 6,6'-dinitro-2,2'-diphenic acid were first experimentally described case, by Christie and Kenner (1922). Atropisomers are stereoisomers arising because of hindered rotation about a single bond, where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual conformers.[1][2] They occur naturally and are important in pharmaceutical d...

Cet article est une ébauche concernant un peintre italien. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Giovanni SerodineNaissance 1594 ou 1600Ascona ou RomeDécès 21 décembre 1630RomeActivités Peintre, plâtrier ou plâtrière, sculpteur, architectemodifier - modifier le code - modifier Wikidata Giovanni Serodine, né en 1600 à Ascona et mort le 21 décembre 1630 à Rome, est un peintre de la période b...

1449 conflict between the Oriats and the Ming dynasty Defense of JingshiMap of Beijing in 1449. Esen's forces were facing the Deshengmen and Xizhimen gates from the North West corner of the map.Date11–17 October 1449LocationBeijingResult Ming victoryBelligerents Northern Yuan (Mongol Khanate) Ming dynastyCommanders and leaders Esen Taishi Jingtai Emperor Yu Qian Strength 70,000 220,000 The Defense of Jingshi (Chinese: 京師保衛戰), also known as the Defense of Beijing (Chinese: ...
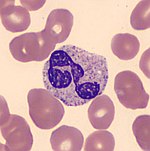
Transfusion of white blood cells A granulocyte transfusion is a medical procedure in which granulocytes (a type of white blood cell) are infused into a person's blood. Granulocyte transfusions were historically used to prevent and treat infections in people with neutropenia (an abnormally low level of neutrophils), but the practice declined in popularity in the 1980s. Interest in the procedure increased in the 1990s due to the development of more effective methods for harvesting granulocytes ...

County in Washington, United States County in WashingtonClallam CountyCountyClallam County Courthouse SealLocation within the U.S. state of WashingtonWashington's location within the U.S.Coordinates: 48°06′45″N 123°26′27″W / 48.1125°N 123.44083333333°W / 48.1125; -123.44083333333Country United StatesState WashingtonFoundedApril 26, 1854SeatPort AngelesLargest cityPort AngelesArea • Total2,671 sq mi (6,920 km2) •&#...

Porträt des David Teniers durch Philip Fruytiers David Teniers (getauft 15. Dezember 1610 in Antwerpen; † 25. April 1690 in Brüssel) war ein flämischer Maler, Zeichner und Kurator. Er war ein äußerst vielseitiger Künstler, der für seine umfangreiche Produktion bekannt ist. Er war ein Erneuerer in einer Vielzahl von Genres wie Historienmalerei, Genremalerei, Landschaft, Porträt und Stillleben. Er ist heute am meisten erinnert als der führende flämische Genremaler seiner Zeit. Tenie...

لمعانٍ أخرى، طالع طيبة (توضيح). طيبةمعلومات عامةصنف فرعي من شعور إيجابي جزء من فضيلة دينيةسبع فضائل جانب من جوانب دينتقمص وجداني ممثلة بـ ابتسامة النقيض قسوة تعديل - تعديل مصدري - تعديل ويكي بيانات طفلين أمريكيين يتشاركان شرب العصير خلال الاحتفال بعيد القيامة. الطيبة...

American baseball player Baseball player Sheriff BlakePitcherBorn: (1899-09-17)September 17, 1899Ansted, West Virginia, U.S.Died: October 31, 1982(1982-10-31) (aged 83)Beckley, West Virginia, U.S.Batted: BothThrew: RightMLB debutJune 29, 1920, for the Pittsburgh PiratesLast MLB appearanceSeptember 26, 1937, for the St. Louis CardinalsMLB statisticsWin–loss record87–102Earned run average4.13Strikeouts621 Teams Pittsburgh Pirates (1920) Chicago Cubs (1924�...

Men's Individual Pursuit competition Cycling Track – Men's individual pursuit at the 2022 Commonwealth GamesVenueLee Valley VeloParkDates30 JulyCompetitors19 from 8 nationsWinning time4:07.760Medalists Aaron Gate New Zealand Thomas Sexton New Zealand Conor Leahy Australia← 20182026 → Cycling at the2022 Commonwealth GamesQualificationRoad cyclingRoad racemenwomenTime trialmenwomenTrack cyclingI...

The Revd KanonJohn PolkinghorneKBE FRSPada 2007Lahir16 Oktober 1930 (umur 93)Weston-super-Mare, InggrisKebangsaanBritania RayaAlmamaterTrinity College, Cambridge (BA dalam bidang matematika (1952), PhD dalam bidang fisika (1955))Westcott House, CambridgePekerjaanFisikawan, imam, penulisDikenal atasFisika partikel; hubungan antara sains dan agamaSuami/istriRuth (née Martin) PolkinghorneAnakPeter (lahir 1957)Isobel(lahir 1959)Michael (1963)Orang tuaGeorge dan Dorothy (née Charlton) ...

ʻĪd al-fiṭrPasto tradizionale in MalesiaNome originaleعيد الفطر Tiporeligiosa Data1 Shawwal Religioneislam Oggetto della ricorrenzafine del digiuno del Ramadan Ricorrenze correlatefine del mese del Ramadan Tradizioni religiosepreghiera, opere di carità, scambio di doni, pasti festivi Tradizioni culinariema'mul Altri nomiʿīd al-ṣaghīr Questa voce sull'argomento islam è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerim...

AeLL.(エール) イオンモール浦和美園にて(2011年6月25日[1])基本情報出身地 日本ジャンル J-POP(アイドル)活動期間 2011年1月1日 - 2014年9月14日事務所 シャイニングウィル公式サイト AeLL. オフィシャルサイトメンバー 西恵利香篠崎愛石條遥梨鷹那空実旧メンバー 戸堂友琴 AeLL.(エール)は、芸能プロダクション・シャイニングウィル所属の日本の女性アイドルグル�...

English economist (1899–1988) For other people with the same name, see William Hutt (disambiguation). This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages) This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: William Harold Hutt – news · n...

Public park in North Yorkshire, England Albert ParkLower LakeTypeUrban ParkLocationMiddlesbroughCoordinates54°33′54″N 1°14′06″W / 54.565°N 1.235°W / 54.565; -1.235Area72 acres (29 ha)Opened11 August 1868 (11 August 1868)AwardsGreen Flag Albert Park is an open access, free public park, located in Middlesbrough, in the borough of Middlesbrough and the ceremonial county of North Yorkshire, England. The park has been granted the Green Flag Award by th...

