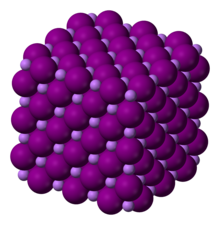
Lithium iodide
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

L'île Howe merupakan salah satu pulau dari archipelago Kerguelen, terletak di utara île Foch, setelah île MacMurdo. Panjangnya sekitar 8 kilometer. Sumber Geoportail - Carte des îles Kerguelen Diarsipkan 2007-11-14 di Wayback Machine. Tableau général de la France outre-mer - Maison de la Géographie Koordinat: 48°52′S 69°27′E / 48.867°S 69.450°E / -48.867; 69.450 Artikel bertopik geografi atau tempat Prancis ini adalah sebuah rintisan. Anda dapat membant...

Unincorporated community in Virginia, US Onemo is an unincorporated community in Mathews County, in the U. S. state of Virginia. Onemo Post Office, 2012 References U.S. Geological Survey Geographic Names Information System: Onemo, Virginia vteMunicipalities and communities of Mathews County, Virginia, United StatesCounty seat: MathewsCDPs Gwynn Mathews Map of Virginia highlighting Mathews CountyUnincorporatedcommunities Bavon Beaverlett Blackwater Blakes Bohannon Cardinal Cobbs Creek Cricket ...
Pour les articles homonymes, voir Bazaar. Harper's BazaarTitre original (en) Harper's BazaarFormat MagazineLangue AnglaisGenre Magazine de mode fémininDate de création 2 novembre 1867Périodicité MensuellePays États-UnisÉditeurs Hearst CorporationJustine Picardie (en)Glenda BaileyISSN 0017-78732692-3815Site web (en) harpersbazaar.comlogomodifier - modifier le code - modifier Wikidata Harper's Bazaar (Harper's Bazar à sa création) est un magazine féminin d'origine américain...

Public limited company and part of Tata Group Tata Oil Mills CompanyCompany typeSubsidiaryFounded10 December 1917; 106 years ago (1917-12-10)FounderDorabji TataDefunct28 December 1994 (1994-12-28)FateAcquired and absorbed by Hindustan Lever LimitedHeadquartersBombay, IndiaParentTata Group Tata Oil Mills Company also known as TOMCO, was a public limited company and part of Tata Group. It was incorporated on 10 December 1917 with head office at Bombay by Dorabji...

Refrancorecomune LocalizzazioneStato Italia Regione Piemonte Provincia Asti AmministrazioneSindacoRoberta Volpato (lista civica Insieme) dal 27-5-2019 TerritorioCoordinate44°56′14″N 8°20′31″E / 44.937222°N 8.341944°E44.937222; 8.341944 (Refrancore)Coordinate: 44°56′14″N 8°20′31″E / 44.937222°N 8.341944°E44.937222; 8.341944 (Refrancore) Altitudine150 m s.l.m. Superficie13,21 km² Abitanti1 551...
1999–2001 armed conflict in Yugoslavia Insurgency in the Preševo ValleyPart of the Yugoslav WarsMap of the Preševo ValleyDate12 June 1999 – 1 June 2001[1](1 year, 11 months, 2 weeks and 6 days)[a]LocationGround Safety Zone and Albanian populated settlements outside it in Preševo, Bujanovac, and Medveđa municipalities, FR YugoslaviaResult Končulj Agreement[2][3][4] Yugoslavia retakes the buffer zone[4] UÇPMB disbande...

Foto satelit dai Pulau Teraina .(NASA) Pulau Teraina atau juga dikenal sebagai Pulau Washington, merupakan sebuah pulau paling utara dari Kepulauan Line, Kiribati. Pulau ini merupakan sebuah atol koral dengan luas sekitar 14.2 kilometer persegi,[1][2] dan memiliki ketinggian rata-rata 2 meter di atas permukaan laut. Pulau ini juga memiliki sebuah danau air tawar yang terletak di ujung bagian barat. Pulau Teraina pertamakali terlihat oleh dunia barat pada tahun 1798; ditemukan ...

Extreme form of authoritarianism Joseph Stalin (left), leader of the Union of Soviet Socialist Republics, and Adolf Hitler (right), leader of the German Reich—considered prototypical dictators of totalitarian regimes, of the left and right respectively Totalitarianism is a political system and a form of government that prohibits opposition political parties, disregards and outlaws the political claims of individual and group opposition to the state, and controls the public sphere and the pr...

Lineage of the Israelite king David House of David redirects here. For other uses, see House of David (disambiguation). House of Davidבית דודParent houseTribe of JudahCountryUnited Kingdom of Israel and JudahKingdom of JudahFounderDavid (traditional)Final rulerZedekiahTitlesKing of IsraelKing of JudahEstate(s)IsraelCadet branchesHouse of Solomon The Davidic line or House of David (Hebrew: בֵּית דָּוִד, romanized: Bēt Dāvīḏ) is the lineage of the Israelite ...

American actor (1917–1997) Robert MitchumMitchum in 1949BornRobert Charles Durman Mitchum(1917-08-06)August 6, 1917Bridgeport, Connecticut, U.S.DiedJuly 1, 1997(1997-07-01) (aged 79)Santa Barbara, California, U.S.Resting placeCremated; Ashes scattered in the Pacific OceanOccupationsActorsingerYears active1942–1997Political partyRepublicanSpouse Dorothy Spence (m. 1940)Children3, including James and Christopher MitchumRelativesJulie Mitchum ...

Hospital in Oregon, United StatesSky Lakes Medical CenterSky Lakes Medical CenterGeographyLocation2865 Daggett Avenue, Klamath Falls, Oregon, United StatesCoordinates42°15′14.52″N 121°47′04.01″W / 42.2540333°N 121.7844472°W / 42.2540333; -121.7844472OrganizationCare systemCommunity-based non-profitTypeGeneral, Surgical, TeachingAffiliated universityOregon Health & Science UniversityServicesEmergency departmentLevel III trauma centerBeds176[1]Hel...

Lokasi County County Cork (bahasa Irlandia: Contae Chorcaí) ialah county terluas di Republik Irlandia. Hampir setengah juta jiwa tinggal di County Cork. Pimpinan Perang Kemerdekaan Irlandia, Michael John Collins, lahir di Clonakilty, County Cork. Ia terbunuh di Béal na mBláth, Cork bagian barat. Cork Barat dikenal akan pemandangannya yang indah. Kota-kota utama di County Cork adalah Cork, Youghal, Mallow, Bandon, Clonakilty, Kinsale, Blarney dan Cobh. County ini dijuluki County Pemberontak...

Badan Urusan Kebudayaan文化庁Bunka-chōGedung UtamaInformasi lembagaDibentuk15 Juni 1968; 56 tahun lalu (1968-06-15)[1]Nomenklatur sebelumnyaBiro Budaya Departemen PendidikanKomite Perlindungan Properti BudayaWilayah hukum JepangKantor pusat85-4 Yabunouchi-cho, Shimochoja-machi-dori, Shinmachi-nishi-iru, Kamigyo-ku, Kyoto-shi, Kyoto 602-8959, Jepang35°1′14″N 135°45′22″E / 35.02056°N 135.75611°E / 35.02056; 135.75611Pegawai301 (202...

Football team of Idaho State University Idaho State Bengals football2024 Idaho State Bengals football team First season1902; 122 years ago (1902)Athletic directorPauline Thiros[1]Head coachCody Hawkins 1st season, 3–8 (.273)StadiumICCU Dome[2](capacity: 12,000)Year built1970Field surfaceSoftTop MatrixLocationPocatello, IdahoNCAA divisionDivision I FCSConferenceBig SkyPast conferencesIndependent (1902–1949, 1961–1962)RMAC (1950–1960)All-time record478&...

عنتالقوات المسلحة اليمنيةالقائد الأعلى: رئيس الجمهورية رشاد محمد العليمي وزير الدفاع: محسن محمد الداعري - رئيس هيئة الأركان العامة: صغير حمود أحمد عزيزالمناطق العسكرية المنطقة الأولى المنطقة الثانية المنطقة الثالثة المنطقة الرابعة المنطقة الخامسة المنطقة السادسة المنط�...

Medical conditionProgressive muscular atrophyOther namesDuchenne–Aran muscular atrophy, othersSpecialtyNeurology Progressive muscular atrophy (PMA), also called Duchenne–Aran disease and Duchenne–Aran muscular atrophy, is a disorder characterised by the degeneration of lower motor neurons, resulting in generalised, progressive loss of muscle function. PMA is classified among motor neuron diseases (MND) where it is thought to account for around 4% of all MND cases.[1] PMA affects...

Chemical compound with isocyanide group (-N+≡C-) Not to be confused with Isocyanate. [ R − N ⊕ ≡ C ⊖ : ⟷ R − N ¨ = C : ] {\displaystyle \left[{\ce {R}}-{\overset {\oplus }{\ce {N}}}{\ce {#}}{\overset {\ominus }{\ce {C}}}{\ce {:\,<->R-{\ddot {N}}=C{:}}}\right]} General resonance structure of an isocyanide An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –N+≡C−. It is the isomer ...

This article's use of external links may not follow Wikipedia's policies or guidelines. Please improve this article by removing excessive or inappropriate external links, and converting useful links where appropriate into footnote references. (November 2023) (Learn how and when to remove this message) New Generation Artists logo BBC Radio 3 New Generation Artists scheme (also known as the NGA scheme) was launched in 1999 by Adam Gatehouse as part of the BBC's commitment to young musical talen...

اضغط هنا للاطلاع على كيفية قراءة التصنيف سعدان العالم القديم المرتبة التصنيفية فصيلة[1][2] التصنيف العلمي فوق النطاق حيويات مملكة عليا أبواكيات مملكة بعديات حقيقية عويلم كلوانيات مملكة فرعية ثانويات الفم شعبة حبليات شعيبة فقاريات...

2017年静岡県知事選挙 2013年 ← 2017年6月25日 (2017-06-25) → 2021年 投票率 46.44% 候補者 川勝平太 溝口紀子 政党 無所属 無所属 得票数 833,389 563,316 得票率 59.67% 40.33% 選挙前知事 川勝平太 無所属 選出知事 川勝平太 無所属 この項目では色を扱っています。閲覧環境によっては、色が適切に表示されていない場合があります。 2017年静岡県知事選挙(2017ねんしず...