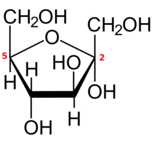
Hexose
|
Read other articles:

Ini adalah nama Mandailing, marganya adalah Lubis. Todung Mulya Lubis Duta Besar Indonesia untuk Norwegia ke-11Masa jabatan20 Februari 2018 – 31 Januari 2023PresidenJoko Widodo PendahuluYuwono A. PutrantoPenggantiTeuku Faizasyah Informasi pribadiLahir4 Juli 1949 (umur 74)Muara Botung, Mandailing Natal, Sumatera Utara, Indonesia[1]PendidikanUniversitas IndonesiaUniversity of California, BerkeleyHarvard Law SchoolSunting kotak info • L • B Prof. Dr. Todung ...

Ibnu Zubair beralih ke halaman ini. Untuk penjelajah dan ahli geografi Arab, lihat Ibnu Jubair. Abdullah bin Zubairعبد الله ابن الزبيرDirham perak bergaya Sasaniyah, dicetak atas nama Abdullah bin Zubair di Fars pada 91 H/690 MKhalifah (diperdebatkan)[a]Berkuasa683–692PendahuluYazid IPenerusAbdul Malik bin MarwanInformasi pribadiKelahiranMei 624 MMadinah, Hijaz, ArabiaKematianOktober/November 692 M (umur 68)Makkah, HijazPemakamanJannatul Mu'alla, MakkahSukuQuraisy (...

العلاقات التشيكية الغانية التشيك غانا التشيك غانا تعديل مصدري - تعديل العلاقات التشيكية الغانية هي العلاقات الثنائية التي تجمع بين التشيك وغانا.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين: وجه المقارنة التشيك غانا �...

This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this article by introducing citations to additional sources.Find sources: Peruvian División Intermedia 1984–1987 – news · newspapers · books · scholar · JSTOR (April 2024) Football leagueDivisión IntermediaFounded1984Folded1987Country PeruConfederationCONMEBOLLevel on pyramid2Promotion toPrimera DivisiónReleg...

كارلو جولدوني (بالإيطالية: Carlo Goldoni) معلومات شخصية اسم الولادة (بالإيطالية: Carlo Osvaldo Goldoni) الميلاد 25 فبراير 1707(1707-02-25)البندقية الوفاة 6 فبراير 1793 (85 سنة)باريس مواطنة جمهورية البندقية مملكة فرنسا المملكة الفرنسية الجمهورية الفرنسية الأولى الحياة العملية المدرسة ال...

English dancer and choreographer (1673–1760) A Collection of Ball-dances Perform'd at Court; all compos'd by Mr. Isaac, and writ down in characters, by John Weaver, dancing-master (1706) John Weaver (baptised 21 July 1673 – 24 September 1760) is widely regarded as the father of English ballet and of English pantomime. He was an English dancer, dancing master and choreographer, and producer of a number of works on dancing. Early life Weaver was born in Shrewsbury where he was baptised on 2...

Video game seriesGeometry WarsGenre(s)Multi-directional shooterDeveloper(s)Bizarre Creations (2003–2010)Kuju Entertainment (2007)Lucid Games (2014)Publisher(s)Microsoft Game Studios (2003–2008)Vivendi Games (2007)Activision (2008–present)Creator(s)Stephen CakebreadPlatform(s)XboxXbox 360Microsoft WindowsWiiNintendo DSiOSLinuxOS XPlayStation 3PlayStation 4Xbox OneAndroidPlayStation VitaFirst releaseGeometry WarsNovember 18, 2003Latest releaseGeometry Wars 3: Dimensions EvolvedOctober 11,...
2020年夏季奥林匹克运动会波兰代表團波兰国旗IOC編碼POLNOC波蘭奧林匹克委員會網站olimpijski.pl(英文)(波兰文)2020年夏季奥林匹克运动会(東京)2021年7月23日至8月8日(受2019冠状病毒病疫情影响推迟,但仍保留原定名称)運動員206參賽項目24个大项旗手开幕式:帕维尔·科热尼奥夫斯基(游泳)和马娅·沃什乔夫斯卡(自行车)[1]闭幕式:卡罗利娜·纳亚(皮划艇)&#...

Citah Afrika timur Acinonyx jubatus raineyi TaksonomiKerajaanAnimaliaFilumChordataKelasMammaliaOrdoCarnivoraFamiliFelidaeGenusAcinonyxSpesiesAcinonyx jubatusSubspesiesAcinonyx jubatus raineyi Heller, 1913 Tata namaSinonim takson Acinonyx jubatus raineyi (Heller, 1913), A. j. ngorongorensis (Hilzheimer, 1913)[1] lbs Citah Afrika timur atau East African cheetah (Acinonyx jubatus jubatus) adalah suatu populasi citah di Afrika Timur. Citah hidup di padang rumput dan padang rumput yang san...

У этого термина существуют и другие значения, см. Елабуга (значения). ГородЕлабугатат. Алабуга Герб 55°46′00″ с. ш. 52°02′00″ в. д.HGЯO Страна Россия Субъект Федерации Татарстан Муниципальный район Елабужский Городское поселение город Елабуга Глава Рустем Нуриев&...
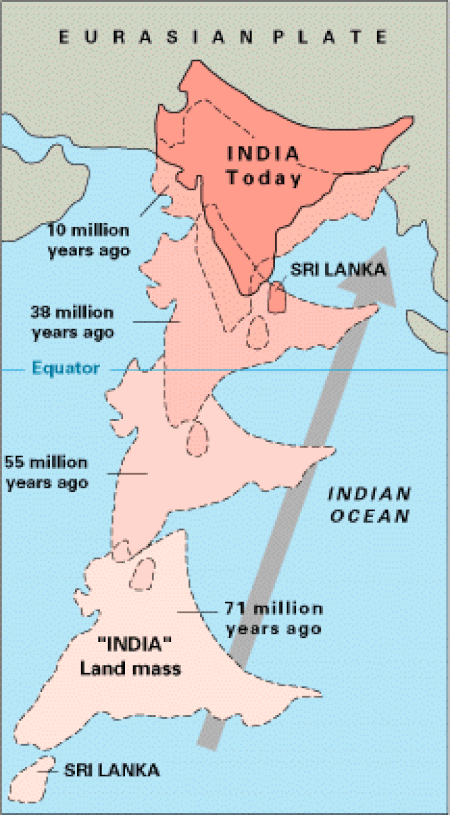
Minor plate that separated from Gondwana This article is about the geological term. For the large plated meal in Indian restaurants, see Thali. Not to be confused with Indian subcontinent. Indian PlateTypeMinorApproximate area11,900,000 km2 (4,600,000 sq mi)[1]Movement1North-eastSpeed126–36 mm/a (1.0–1.4 in/year)[citation needed]FeaturesIndian subcontinent, Indian Ocean, Arabian Sea, Himalayas1Relative to the African Plate The Indian Plate (or Indi...

تجمع عضبات - قرية - تقسيم إداري البلد اليمن المحافظة محافظة حضرموت المديرية مديرية غيل بن يمين العزلة عزلة غيل بن يمين السكان التعداد السكاني 2004 السكان 4 • الذكور 2 • الإناث 2 • عدد الأسر 3 • عدد المساكن 3 معلومات أخرى التوقيت توقيت اليمن (+3 غرين...

Armed wing of the Communist Party of the Philippines This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article relies excessively on references to primary sources. Please improve this article by adding secondary or tertiary sources. Find sources: New People's Army – news · newspapers · books · scholar · JSTOR (February 2021) (Learn how and when ...

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (ديسمبر 2016) منظمة الطاقة الجديدة وتطوير التقنيات الصناعيةالشعارالتاريخالتأسيس 1 أكتوبر 2003 — 1 أكتوبر 1980[1] — 1980[2] الإطارالنوع مؤسسة حكوميةIndependent Administrative Institutio...

Species of bee Xylocopa frontalis Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Arthropoda Class: Insecta Order: Hymenoptera Family: Apidae Genus: Xylocopa Species: X. frontalis Binomial name Xylocopa frontalis(Olivier, 1789) Distribution Native range Xylocopa frontalis, also known by its common name ridge-browed carpenter, is a species of carpenter bee (genus Xylocopa). Description X. frontalis is part of a group of solitary bees call...

Capital of Liège province, Belgium Liege redirects here. For other uses, see Liege (disambiguation). You can help expand this article with text translated from the corresponding article in Chinese. (June 2023) Click [show] for important translation instructions. Machine translation, like DeepL or Google Translate, is a useful starting point for translations, but translators must revise errors as necessary and confirm that the translation is accurate, rather than simply copy-pasting mach...

Imperial circle of the Holy Roman Empire This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages) This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. Please help improve this article by introducing more precise citations. (February 2024) (Learn how and when to remove this message) You can help expand this article ...

This article is about the former station in Norfolk. For the former station in the Isle of Man, see St. Germain's railway station. Former railway station in Norfolk, England St Germain'sLevel crossing very close to the site of St Germain's station.General informationLocationWiggenhall St. Germans, NorfolkEnglandGrid referenceTF616138Other informationStatusDisusedHistoryOriginal companyLynn and Ely Railway[1]Key dates27 Oct 1846Opened[1]Oct 1850Closed St. Germain's railway stat...

Extinct Indo-European language ThracianRegionBulgaria, European Turkey, parts of Southern Serbia, parts of the region of Macedonia (including Paeonia), regions in Northern Greece, parts of Romania, parts of Bithynia in Anatolia. Probably also spoken in parts of Dardania.Extinct6th century AD[1]Language familyIndo-European Daco-Thracian (?)ThracianWriting systemGreekLanguage codesISO 639-3txhLinguist ListtxhGlottologthra1250 Part of a series onIndo-European topics Languages List of Ind...

German art book publisher For other uses, see Taschen (disambiguation). This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages) A major contributor to this article appears to have a close connection with its subject. It may require cleanup to comply with Wikipedia's content policies, particularly neutral point of view. Please discuss further on the talk page. (September 2017) (Learn how and when to remove...



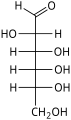
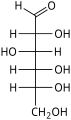





![D-Psicose[8]](http://upload.wikimedia.org/wikipedia/commons/thumb/7/7c/DPsicose_Fischer.svg/62px-DPsicose_Fischer.svg.png)
![D-Fructose[9]](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e5/D-Fructose.svg/72px-D-Fructose.svg.png)
![D-Sorbose[10]](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d6/DSorbose_Fischer.svg/70px-DSorbose_Fischer.svg.png)
![D-Tagatose[11]](http://upload.wikimedia.org/wikipedia/commons/thumb/6/6c/DTagatose_Fischer.svg/70px-DTagatose_Fischer.svg.png)