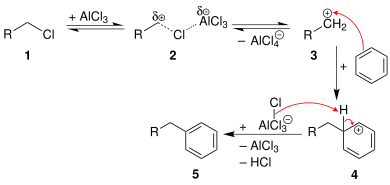
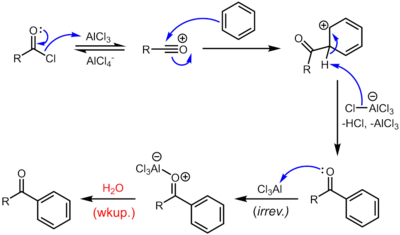
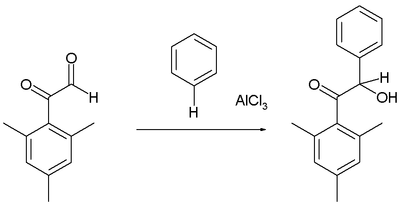
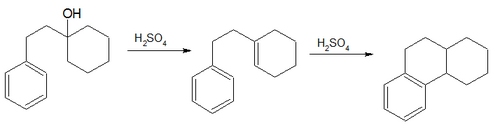
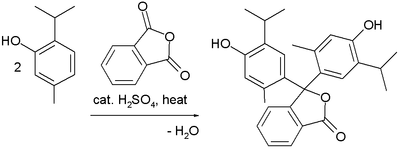
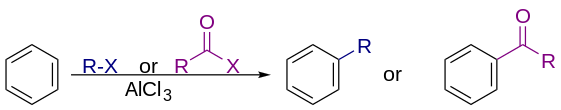Friedel–Crafts reaction
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:
Copparo commune di Italia Tempat categoria:Articles mancats de coordenades Negara berdaulatItaliaRegion di ItaliaEmilia-RomagnaProvinsi di ItaliaProvinsi Ferrara NegaraItalia Ibu kotaCopparo PendudukTotal15.673 (2023 )GeografiLuas wilayah157,01 km² [convert: unit tak dikenal]Ketinggian7 m Berbatasan denganRiva del Po (en) Ferrara Tresignana (en) Jolanda di Savoia Informasi tambahanKode pos44034 Zona waktuUTC+1 UTC+2 Kode telepon0532 ID ISTAT038007 Kode kadaster ItaliaC980 Lain-lai...

Cristiano Del Grosso Informasi pribadiTanggal lahir 24 Maret 1983 (umur 40)Tempat lahir Giulianova, ItaliaTinggi 175 m (574 ft 2 in)Posisi bermain Bek kiriInformasi klubKlub saat ini Bari(pinjaman dari Atalanta)Nomor 3Karier senior*Tahun Tim Tampil (Gol)2000–2006 Giulianova 132 (7)2005–2006 → Ascoli (loan) 30 (0)2006–2008 Cagliari 39 (0)2008–2013 Siena 133 (3)2013– Atalanta 45 (1)2015– → Bari (loan) 0 (0) * Penampilan dan gol di klub senior hanya dihitung ...

U.S. Army's branch for personnel service support and human resources Adjutant General's Corpsbranch insigniaActive16 June 1775Country United StatesBranchU.S. ArmyTypeAdjutant GeneralRolePersonnelHome stationFort Jackson, South CarolinaMotto(s)Defend and ServeBranch colorDark Blue and Scarlet piping CommandersChief of the AG CorpsCOL Chesley D. ThigpenThe Adjutant General of the U.S. ArmyBrigadier General Gregory S. JohnsonU.S Army Deputy Chief of Staff, G-1Lieutenant General Douglas F. S...

American slapstick comedy trio For other uses, see The Three Stooges (disambiguation). This article may require copy editing for grammar, style, cohesion, tone, or spelling. You can assist by editing it. (January 2024) (Learn how and when to remove this template message) The Three StoogesThe Three Stooges in 1937: (clockwise from left) Larry Fine, Curly Howard, and Moe HowardMediumVaudeville, film, televisionNationalityAmericanYears active1922–1970GenresFarce, slapstick, musical comedyForme...

Peeni HenareHenare pada 2019 Menteri Pertahanan ke-41PetahanaMulai menjabat 6 November 2020Perdana MenteriJacinda ArdernPendahuluRon MarkPenggantiPetahanaMenteri Whānau Ora ke-3PetahanaMulai menjabat 26 Oktober 2017Perdana MenteriJacinda ArdernPendahuluTe Ururoa FlavellPenggantiPetahanaMenteri Pemuda ke-14Masa jabatan26 Oktober 2017 – 6 November 2020Perdana MenteriJacinda ArdernPendahuluNikki KayePenggantiPriyanca RadhakrishnanMenteri Pertahanan Sipil ke-26Masa jabatan27 J...

Laboratory in Idaho Falls, Idaho, United States Idaho National LaboratoryMottoThe energy of innovationEstablished1949Research typenuclear energy, national security, energy, and environmentBudgetapprox. $1 billion (2010)DirectorJohn WagnerStaffapprox. 5,700 (2023)LocationIdaho Falls, Idaho, U.S.& a large area to the westCampus890 sq mi (2,310 km2)Operating agencyBattelle Energy AllianceWebsitewww.inl.govFormer Names:INEEL, INEL, ERDA, NRTS INLclass=notpageimage| Locatio...

Ship that carries cargo in intermodal containers Two Maersk Line container ships Class overview NameContainer ship Subclasses (1) Geared or gearless (as per cargo-handling type) (2) Freighter or pure container (as per passenger carrier-type) (3) Feeder or world-wide foreign-going vessel (as per trade) (4) Panamax or post-Panamax vessel (as per breadth of vessel < or > than 32.2m respectively) Built1956–present In service9,535 ships as of 2010[1] General characteristics Propuls...

Scottish amateur golfer (1874–1959) Findlay S. DouglasDouglas on a 1910 tobacco card.Personal informationFull nameFindlay Small DouglasBorn(1874-11-17)17 November 1874St Andrews, ScotlandDied29 March 1959(1959-03-29) (aged 84)Sporting nationality ScotlandCareerCollegeUniversity of St AndrewsStatusAmateurBest results in major championships(wins: 1)U.S. Open8th: 1903The Open ChampionshipDNPU.S. AmateurWon: 1898British AmateurR256: 1913, 1920Achievements and awardsBob Jones Award1959...

House-museum of Aljustrel A street of Aljustrel Aljustrel is a hamlet on the outskirts of Fátima, Portugal, in the municipality of Ourém. It was the birthplace of Lúcia dos Santos, and Francisco and Jacinta Marto, known worldwide as the three little shepherds of Fátima or the three child seers,[1] and the setting for some of the events during the apparitions of Our Lady of Fátima.[2] There is now a small house-museum there. See also Chapel of the Apparitions Parish Church...

Musical instrument This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Archlute – news · newspapers · books · scholar · JSTOR (January 2024) (Learn how and when to remove this message) ArchluteArchlute by Matteo Sellas, 17th CenturyClassification Necked bowl lutes String instruments Related instruments List Ang�...

علم الفلك الراديويمصفوف المراصد العظيم VLA في نيومكسيكو بالولايات المتحدة يقيس الموجات الراديوية الآتية من أماكن كثيرة في الكون.صنف فرعي من علم فلك رصدي يمتهنه radio astronomer (en) الموضوع جرم فلكي تعديل - تعديل مصدري - تعديل ويكي بيانات علم الفلك الكَاشُوفِي[1] أو علم الفلك الإش...

For the Cricketer, see Robert Terry (cricketer). Welsh professional wrestler Rob TerryTerry in 2010Birth nameRobert TerryBorn (1980-04-30) 30 April 1980 (age 44)Swansea, Wales, United Kingdom[1][2]Alma materNeath Port Talbot CollegeSpouse(s)Sarah Treviso (m. 2008)Professional wrestling careerRing name(s)Big Rob[3]The FreakRob Terry[3]Robbie T[4]Billed height6 ft 5 in (1.96 m)[1][5]Billed weight299 lb (136 kg...

قرية بيري الإحداثيات 42°43′01″N 78°00′16″W / 42.716944444444°N 78.004444444444°W / 42.716944444444; -78.004444444444 [1] تاريخ التأسيس 1830 تقسيم إداري البلد الولايات المتحدة[2] التقسيم الأعلى مقاطعة وايومينغ خصائص جغرافية المساحة 6.342748 كيلومتر مربع6.342758 كيلومتر مربع (1 أ...

Island in New York City For other uses, see Hart Island (disambiguation). Hart IslandAerial view of Hart Island, in 2012Location in New York CityGeographyLocationLong Island SoundCoordinates40°51′9″N 73°46′12″W / 40.85250°N 73.77000°W / 40.85250; -73.77000ArchipelagoPelham IslandsArea131.22 acres (53.10 ha)Length1.0 mi (1.6 km)Width0.33 mi (0.53 km)StateNew YorkCityNew York CityBoroughThe BronxAdditional informationTime zoneEastern ...

Physical sensations caused in the mouth by food or drink For the album, see Mouthfeel (album). A child bites into a watermelon, experiencing mouthfeel sensations such as juiciness Mouthfeel refers to the physical sensations in the mouth caused by food or drink, making it distinct from taste. It is a fundamental sensory attribute which, along with taste and smell, determines the overall flavor of a food item.[1][2] Mouthfeel is also sometimes referred to as texture.[2] ...

Medication of the opioid type, patented 1972 Tramadol Clinical dataPronunciationtra' ma doll Trade namesUltram, Zytram, Ralivia, others[1]AHFS/Drugs.comMonographMedlinePlusa695011License data US DailyMed: Tramadol Pregnancycategory AU: C[2] DependenceliabilityLow–moderate[3]Routes ofadministrationBy mouth, intravenous (IV), intramuscular (IM), rectalDrug classOpioid analgesic[4]ATC codeN02AX02 (WHO) N02AJ13 (WHO)Legal status...

XXVIII campionati europei di atletica leggera indoor2005 European Athletics Indoor Championships Competizione Campionati europei di atletica leggera indoor Sport Atletica leggera Edizione 28ª Organizzatore European Athletic Association Date 4-6 marzo 2005 Luogo Madrid, Spagna Partecipanti 563 atleti Nazioni 41 Discipline 28 Impianto/i Palacio de Deportes Statistiche Miglior nazione Russia Il Palacio de Deportes di Madrid Cronologia della competizione Vienna 2002 Birmingham ...
此條目介紹現正廣播的電視頻道。此頁面介紹陕西广播电视台的電視頻道,內容可能會隨农林卫视的現況而有所變更,並增加更多有效資訊。維基百科不是不经筛选的信息的收集处。請留心記載正確資訊,在情報相對明朗之後進行編輯更新。 农林卫视国家/地区 中国大陆所有者陕西广播电视台画面格式SDTV (576i 16:9)语言普通话类别农业播出地区全国总部地点 中国陕西省�...

FIBA EuroBasket Women 1960 Sport Pallacanestro Zona FIBAFIBA Europe Paese ospitante Bulgaria Periodo3 - 11 giugno Squadre10 (da 10 federazioni) Campi1 (in 1 città) Podio Unione Sovietica (5º titolo) Bulgaria Cecoslovacchia Partite giocate35 Il 7º Campionato Europeo Femminile di Pallacanestro FIBA si è svolto a Sofia, in Bulgaria dal 3 al 11 giugno 1960. Indice 1 Risultati 1.1 Turno preliminare 1.1.1 Gruppo A 1.1.2 Gruppo B 1.2 Classificazione 7º-10º posto 1.3 Classif...

Newspaper in Chico, California Chico Enterprise-RecordTypeDaily newspaperFormatBroadsheetOwner(s)Digital First MediaPublisherJim GleimEditorMike WolcottFoundedNov. 12, 1853 (as the Butte Record)Headquarters400 E. Park Ave. Chico, CaliforniaUnited StatesCirculationunder 10,000[1]ISSN0746-5548Websitechicoer.com The Chico Enterprise-Record is the daily newspaper of Chico, California. Also known as the E-R, the newspaper was first published in Bidwell Bar, California as the Butte Record i...