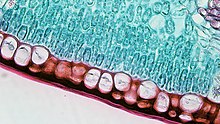
Cutinase
| |||||||||||||||||||||||||||||
Read other articles:

Paus Aleksander VIII (1610–91). Paus Aleksander VIII (menjabat 1689–1691) mengangkat 14 kardinal dalam tiga konsistori. 7 November 1689 Pietro Ottoboni (1667–1740) Pietro Ottoboni 13 Februari 1690 Ferdinando d'Adda (1650–1719) Bandino Panciatici Giacomo Cantelmo Ferdinando d'Adda Toussaint de Forbin-Janson Giambattista Rubini Francesco del Giudice Giambattista Costaguti Carlo Bichi Giuseppe Renato Imperiali Luigi Omodei Gian Francesco Albani 13 November 1690 Francesco Barberini (1662�...

Pour les articles homonymes, voir Cromwell. Oliver Cromwell Portrait d'Oliver Cromwell par Samuel Cooper. Fonctions Lord-protecteur du Commonwealth d'Angleterre, d'Écosse et d'Irlande 16 décembre 1653 – 3 septembre 1658(4 ans, 8 mois et 18 jours) Prédécesseur Fonction crééeConseil d'État Successeur Richard Cromwell Biographie Date de naissance 25 avril 1599 Lieu de naissance Huntingdon (Angleterre) Date de décès 3 septembre 1658 (à 59 ans) Lieu de décès Lond...

The Right ReverendGérard Calvet, O.S.B.Abbot of Le Barroux AbbeyAppointed2 July 1989Term ended25 November 2003SuccessorDom Louis-Marie de Geyer d'OrthOrdersOrdination13 May 1956Personal detailsBornGérard Calvet(1927-11-18)18 November 1927Bordeaux, FranceDied28 February 2008(2008-02-28) (aged 80)NationalityFrenchDenominationRoman CatholicMottoPer Te VirgoCoat of arms Dom Gérard Calvet (18 November 1927 – 28 February 2008) was a French Catholic abbot and founder of the Sainte Madelein...

Dominican-American baseball pitcher (born 1988) In this Spanish name, the first or paternal surname is Chapman and the second or maternal family name is de la Cruz. Baseball player Aroldis ChapmanChapman with the New York Yankees in 2016Pittsburgh Pirates – No. 45PitcherBorn: (1988-02-28) February 28, 1988 (age 36)Holguín, CubaBats: LeftThrows: LeftMLB debutAugust 31, 2010, for the Cincinnati RedsMLB statistics (through April 15, 2024)Win–loss record50–41Ear...

NetflixTangkapan layar situs web bahasa Inggris Netflix tahun 2019URLnetflix.com TipePlatform aliran OTTPerdagangan ?YaRegistration (en)DiperlukanLangue Daftar Arab (Mesir dan Baku Modern)Basque (hanya konten) Katalan (hanya konten) Tionghoa (Kanton dan Mandarin)KroasiaCekoDenmarkBelandaInggris (Amerika Serikat dan Britania)FilipinoFinlandiaPrancisJermanYunaniIbraniHindi[1]HungariaIndonesia[2]ItaliaJepangKannadaKoreaMelayuMalayalamMarathi (hanya konten)Norwegia (Bokmål)P...

Country in Central America For other uses, see Honduras (disambiguation). Republic of HondurasRepública de Honduras (Spanish) Flag Coat of arms Motto: Libre, Soberana e IndependienteFree, Sovereign and IndependentAnthem: Himno Nacional de HondurasNational Anthem of HondurasCapitaland largest cityTegucigalpa14°6′N 87°13′W / 14.100°N 87.217°W / 14.100; -87.217Official languagesSpanishEthnic groups (2016)[1]90% Mestizo (mixed Indi...

Pour les articles homonymes, voir Ancien 6e arrondissement de Paris et 6e arrondissement. 6e arrondissement de Paris « arrondissement du Luxembourg » La place Saint-Sulpice avec l'église. Administration Pays France Ville Paris Quartiers administratifs Monnaie (21)Odéon (22)Notre-Dame-des-Champs (23)Saint-Germain-des-Prés (24) Maire Mandat Jean-Pierre Lecoq (LR) depuis 1994 Code postal 75006 Code Insee 75106 Démographie Population 40 209 hab. (2021 ) Densité 18...

Village in Albania Municipal unit in Vlorë, AlbaniaShushicëMunicipal unitShushicëCoordinates: 40°32′N 19°32′E / 40.533°N 19.533°E / 40.533; 19.533Country AlbaniaCountyVlorëMunicipalityVlorëPopulation (2011) • Municipal unit3,981Time zoneUTC+1 (CET) • Summer (DST)UTC+2 (CEST) Shushicë is a village and a former municipality in the Vlorë County, southwestern Albania. At the 2015 local government reform it became a subdivisi...

North Korean politician (1920–2003) In this Korean name, the family name is Choe. Choe In-dok최인덕LeaderKim Il Sung Kim Jong Il Personal detailsBorn1920Anju, South Pyongan, Kankyōhoku-dō, Korea, Empire of JapanDied31 August 2003(2003-08-31) (aged 82–83)Pyongyang, North KoreaCitizenshipNorth KoreanNationalityKoreanPolitical partyWorkers' Party of KoreaMilitary serviceAllegiance North KoreaBranch/service Korean People's ArmyRank Ch'asu (Vice Marshal) Choe In-dok (Korean:...

Locomotora Henschel del Tren de Arganda, en la laguna del Campillo. La ARGANDA a 20 km/h. Circulando en la antigua línea del Ferrocarril del Tajuña. Doble circulación del Tren de Arganda. Apeadero de la Laguna del Campillo (Rivas-Vaciamadrid).La locomotora Arganda, también denominada locomotora Henschel Arganda,[1] es una locomotora de vapor-carbón que fue fabricada en el año 1925 en Kassel por la empresa alemana Henschel. A lo largo de su vida operativo estuvo destinada en el pu...

Handheld game console Anbernic RG351ManufacturerAnbernicTypeHandheld game consoleGenerationEighthRelease date September 2020 (RG351P) January 2021 (RG351M) February 2021 (RG351V) Operating systemLinuxCPURockchip RK3326 @ 1.5GHzMemory1GBRemovable storageSD card The Anbernic RG351 is a Linux-based handheld game console created in China by Anbernic. The console uses a microSD card for storage and is a digital ROM-only console. It is the successor to the RG350, and has emerged as a prominent hand...

Work People's College as it appeared in 1913. Note the parallel American and red flags flying over the building. Work People's College (Finnish: Työväen Opisto) was a radical labor college (a type of a folk high school governed by the worker's movement) established in Smithville (Duluth), then a suburb of Duluth, Minnesota, in 1907 by the Finnish Socialist Federation of the Socialist Party of America. School administrators and faculty were sympathetic to the syndicalist left wing of the Fin...

Devil May Cry 5 Diterbitkan di8 Maret 2019GenreAction-adventure, hack and slashBahasa Daftar Inggris, Italia, Jepang, Jerman, Korea, Polandia, Portugis Brasil, Prancis, Rusia, Spanyol, Tionghoa Sederhana dan Tionghoa Tradisional 60 Karakteristik teknisPlatformWindows, PlayStation 4 dan Xbox One MesinRE Engine (en) ModePermainan video pemain tunggal Formatcakram optis dan distribusi digital Metode inputpapan tombol komputer, tetikus dan gamepad Informasi pengembangPengembangCapcomPenyuntingCap...

Neighbourhood in Toronto, Ontario, CanadaSouth Core SocoNeighbourhoodView of South Core looking east from Bremner Boulevard and Lower Simcoe StreetSouth CoreLocation in TorontoCoordinates: 43°38′38″N 79°22′46″W / 43.64389°N 79.37944°W / 43.64389; -79.37944Country CanadaProvince OntarioCityToronto South Core is a neighbourhood located in downtown Toronto, Ontario, Canada. The South Core occupies the eastern portions of the Railway Lands. The remod...

State of Malaysia This article is about the Malaysian state. For the computer scientist, see Alan Perlis. Indera Kayangan redirects here. For the state constituency, see Indera Kayangan (state constituency). StatePerlis PeghelihStatePerlis Indera KayanganOther transcription(s) • Jawiڤرليس • Chinese玻璃市 • Tamilபெர்லிஸ்Perlis (Transliteration) • Thaiปะลิส FlagCoat of armsNickname(s): Perlis Inde...

关于与「孫中山」標題相近或相同的条目页,請見「孫中山 (消歧義)」。 孫中山孙中山标准像(摄于1922年11月15日) 中華民國臨時大總統任期1912年1月1日—1912年4月1日 副总统黎元洪前任無(首任)继任袁世凯 中國國民黨總理任期1919年10月10日—1925年3月12日 前任首任继任逝世後永久保留總理職銜[1]張靜江(中國國民黨中央執行委員會主席) 中華�...

Saxifraga Saxifraga aizoides Klasifikasi ilmiah Kerajaan: Plantae Klad: Tracheophyta Klad: Angiospermae Klad: Eudikotil Ordo: Saxifragales Famili: Saxifragaceae Genus: SaxifragaTourn. ex L. Spesies Lihat teks Sinonim[1] Boecherarctica Á.Löve Cascadia A.M.Johnson Micranthes Haw. Zahlbrucknera Rchb. Saxifraga adalah genus tumbuhan terbesar dalam famili Saxifragaceae, yang berisi sekitar 465 spesies tanaman tahunan holarktika.[2][3] Kata Latin saxifraga secara harfiah ...

Nayib Bukele Retrato oficial, 2019. 60.º y 62.º presidente de la República de El Salvador Actualmente en el cargo Desde el 1 de junio de 2024Gabinete Gabinete de Nayib BukeleVicepresidente Félix UlloaPredecesora Claudia Rodríguez 1 de junio de 2019-1 de diciembre de 2023 [nota 1]Gabinete Gabinete de Nayib BukeleVicepresidente Félix UlloaPredecesor Salvador Sánchez CerénSucesora Claudia RodríguezDesignada por el presidente de la República, encargada del Despacho Presidente pro...

Questa voce o sezione sugli argomenti vescovi italiani e religiosi italiani non cita le fonti necessarie o quelle presenti sono insufficienti. Puoi migliorare questa voce aggiungendo citazioni da fonti attendibili secondo le linee guida sull'uso delle fonti. Segui i suggerimenti dei progetti di riferimento 1, 2. Cosma Migliorati Orsini, O.S.B.cardinale di Santa Romana Chiesa Incarichi ricoperti Arcivescovo metropolita di Trani (1478-1481) Cardinale presbitero di San Sisto (1480) C...

Welsh singer (born 1950) Mary HopkinHopkin at the Eurovision Song Contest 1970Background informationBorn (1950-05-03) 3 May 1950 (age 74)[1]Pontardawe, WalesGenresFolk popProgressive rockOccupationsSingersongwriterInstrumentsVocalsguitarYears active1968–presentLabelsAppleManticoreClanNumero Uno [it]Mary Hopkin MusicSpouse Tony Visconti (m. 1971; div. 1981)Websitemaryhopkin.comMusical artist Mary Hopkin (born 3 May...