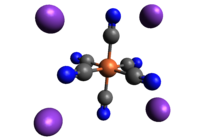
Potassium ferrocyanide
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Arondisemen Bernay Administrasi Negara Prancis Region Haute-Normandie Departemen Eure Kanton 15 Komune 239 Sous-préfecture Bernay Statistik Luas¹ 2,060 km² Populasi - 1999 131,050 - Kepadatan 64/km² Lokasi Lokasi Bernay di Haute-Normandie ¹ Data Pendaftaran Tanah Prancis, tak termasuk danau, kolam, dan gletser lebih besar dari 1 km² (0.386 mi² atau 247 ekar) juga muara sungai. Arondisemen Bernay merupakan sebuah arondisemen di Prancis, terletak di département Eure...

Часть серии статей о Холокосте Идеология и политика Расовая гигиена · Расовый антисемитизм · Нацистская расовая политика · Нюрнбергские расовые законы Шоа Лагеря смерти Белжец · Дахау · Майданек · Малый Тростенец · Маутхаузен ·&...

Institut national des hautes études de la sécurité et de la justiceHistoireFondation 28 octobre 2009Dissolution 31 décembre 2020Prédécesseur Institut national des hautes études de sécurité (d)Successeur Institut des hautes études du ministère de l'IntérieurCadreForme juridique Établissement public national à caractère administratifDomaine d'activité Enseignement supérieurSiège Paris (1, 75700)Pays FranceOrganisationPrésidents Michel Cadot (depuis 2010), Jacques Buisso...

Об экономическом термине см. Первородный грех (экономика). ХристианствоБиблия Ветхий Завет Новый Завет Евангелие Десять заповедей Нагорная проповедь Апокрифы Бог, Троица Бог Отец Иисус Христос Святой Дух История христианства Апостолы Хронология христианства Ран�...

Main article: 2008 Summer Olympics venues The Bicycle Moto Cross Field at Laoshan. The Laoshan Bicycle Moto Cross (BMX) (simplified Chinese: 老山小轮车赛场; traditional Chinese: 老山小輪車賽場; pinyin: Lǎoshān Xiǎolúnchē Sàichǎng) was one of 9 temporary venues used for the 2008 Summer Olympics. It was located in Laoshan, Shijingshan District, Beijing. The venue was used for the men's and women's BMX racing events. The arena has in recent years been transformed...

Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. Certaines informations figurant dans cet article ou cette section devraient être mieux reliées aux sources mentionnées dans les sections « Bibliographie », « Sources » ou « Liens externes » (novembre 2008). Vous pouvez améliorer la vérifiabilité en associant ces informations à des références à l'aide d'appels de notes. Pour les articles homonymes, voir Famille de Ment...

Elements and compounds that are readily vaporized For other uses, see Volatile (disambiguation). This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this article by introducing citations to additional sources.Find sources: Volatile astrogeology – news · newspapers · books · scholar · JSTOR (October 2011) Volatiles are the group of chemical elements and chemical compound...

Questa voce o sezione sull'argomento calciatori spagnoli non cita le fonti necessarie o quelle presenti sono insufficienti. Puoi migliorare questa voce aggiungendo citazioni da fonti attendibili secondo le linee guida sull'uso delle fonti. Segui i suggerimenti del progetto di riferimento. Questa voce sugli argomenti allenatori di calcio spagnoli e calciatori spagnoli è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti dei progett...

Lukas 1Permulaan Injil Lukas (pasal 1:1-7a), folio 102 pada Minuscule 481, yang dibuat sekitar abad ke-10.KitabInjil LukasKategoriInjilBagian Alkitab KristenPerjanjian BaruUrutan dalamKitab Kristen3← Markus 16 pasal 2 → Lukas 1 (disingkat Luk 1) adalah pasal pertama dari Injil Lukas pada Perjanjian Baru dalam Alkitab Kristen. Disusun oleh Lukas, seorang Kristen yang merupakan teman seperjalanan Rasul Paulus.[1][2] Pasal yang terdiri dari 80 ayat ini merupakan salah...

Software development kit for iOS iOS SDK(iOS Software Development Kit)LogoScreenshot iOS SDK 9.1 included in Xcode 7.1.1Developer(s)Apple Inc.Initial releaseMarch 6, 2008; 16 years ago (2008-03-06)Operating systemmacOSPlatformiOS, iPadOSAvailable inEnglishTypeSoftware development kitWebsiteApple Developer The iOS SDK (iOS Software Development Kit), formerly the iPhone SDK, is a software development kit (SDK) developed by Apple Inc. The kit allows for the development of mobil...

This article has an unclear citation style. The references used may be made clearer with a different or consistent style of citation and footnoting. (January 2024) (Learn how and when to remove this template message) Karl Trautmann (born 26 April 1932) is an East German former football manager and footballer who last managed SpVg Aurich. Career Trautmann helped East German side 1. FC Frankfurt reach the final of the 1976 FDGB-Pokal and helped East German side Eisenhüttenstädter FC Stahl rea...

追晉陸軍二級上將趙家驤將軍个人资料出生1910年 大清河南省衛輝府汲縣逝世1958年8月23日(1958歲—08—23)(47—48歲) † 中華民國福建省金門縣国籍 中華民國政党 中國國民黨获奖 青天白日勳章(追贈)军事背景效忠 中華民國服役 國民革命軍 中華民國陸軍服役时间1924年-1958年军衔 二級上將 (追晉)部队四十七師指挥東北剿匪總司令部參謀長陸軍�...

Gentle politeness and courtly manners Courtly redirects here. For court culture in medieval to early modern Europe, see Courtly love and Royal court. Not to be confused with Etiquette. Courtesy (from the word courteis, from the 12th century) is gentle politeness and courtly manners. In the Middle Ages in Europe, the behaviour expected of the nobility was compiled in courtesy books. History The apex of European courtly culture was reached in the Late Middle Ages and the Baroque period (i.e. ro...

This is a list of big-box stores by country. Multi-national Auchan - hypermarkets; France B&Q - DIY home improvement; United Kingdom Babies R Us - baby clothes, care products, furniture, toys (Defunct) Barnes & Noble - books, music, videos, magazines Best Buy - music, videos, electronics, computer software, appliances Blockbuster Video - video rental (Defunct) Borders - books, music, videos (Defunct) Bricorama - D.Y, gardening; France Bunnings - Home improvement; Australia and New Ze...

Fictional character in the Star Wars franchise For the television series, see Obi-Wan Kenobi (miniseries). Fictional character Obi-Wan KenobiStar Wars characterAlec Guinness as Obi-Wan Kenobi in Star Wars: Episode IV – A New Hope (1977)First appearanceStar Wars (1977)Created byGeorge LucasBased onGeneral Makabe Rokurōtaby Akira KurosawaPortrayed by Alec Guinness (Episodes IV–VI) Ewan McGregor (Episodes I–III, Obi-Wan Kenobi) Voiced byas Ben Kenobi: Stephen Stanton (Star Wars: Battlefro...

Sceaux 行政国 フランス地域圏 (Région) イル=ド=フランス地域圏県 (département) オー=ド=セーヌ県郡 (arrondissement) アントニー郡小郡 (canton) 小郡庁所在地INSEEコード 92071郵便番号 92330市長(任期) フィリップ・ローラン(2008年-2014年)自治体間連合 (fr) メトロポール・デュ・グラン・パリ人口動態人口 19,679人(2007年)人口密度 5466人/km2住民の呼称 Scéens地理座標 北緯48度4...
2020年夏季奥林匹克运动会波兰代表團波兰国旗IOC編碼POLNOC波蘭奧林匹克委員會網站olimpijski.pl(英文)(波兰文)2020年夏季奥林匹克运动会(東京)2021年7月23日至8月8日(受2019冠状病毒病疫情影响推迟,但仍保留原定名称)運動員206參賽項目24个大项旗手开幕式:帕维尔·科热尼奥夫斯基(游泳)和马娅·沃什乔夫斯卡(自行车)[1]闭幕式:卡罗利娜·纳亚(皮划艇)&#...

British biochemist Peter MitchellFRCSBornPeter Dennis Mitchell(1920-09-29)29 September 1920[1]Mitcham, Surrey, EnglandDied10 April 1992(1992-04-10) (aged 71)Bodmin, Cornwall, EnglandAlma materUniversity of Cambridge (BA, MA, PhD)Known forDiscovery of the mechanism of ATP synthesisAwards FRS (1974)[1] Rosenstiel Award (1976) Nobel Prize in Chemistry (1978) Sir Hans Krebs Medal (1978) Copley Medal (1981) Scientific careerFieldsBiochemistryInstitutions University o...

Territory of Canada This article is about the contempary Canadian territory. For its predecessor, see North-Western Territory. For the former U.S. territory, see Northwest Territory. For similar names, see Northwest (disambiguation). Territory in CanadaNorthwest Territories Territoires du Nord-Ouest (French)[1]Territory FlagCoat of arms BC AB SK MB ON QC NB PE NS NL YT NT NU Coordinates: 67°N 121°W / 67°N 121°W / 67; -121[2]CountryCanadaBefore ...

يفتقر محتوى هذه المقالة إلى الاستشهاد بمصادر. فضلاً، ساهم في تطوير هذه المقالة من خلال إضافة مصادر موثوق بها. أي معلومات غير موثقة يمكن التشكيك بها وإزالتها. (ديسمبر 2018) هذه قائمة الكواكب الصغيرة المرقمة في النظام الشمسي من 47001 - إلي -48000 قريبة من الأرض الح�...