Morris S. Kharasch
| |||||||||||||||||||||||||||||||||
Read other articles:

MINI La Mini nuova in confronto con l'antenata Descrizione generale Costruttore Mini Tipo principale berlina 2 volumi Produzione dal 2001 Sostituisce la Mini (1959) Altre caratteristiche Altre antenate Rover serie 100 La Mini è un'automobile, rivisitazione in chiave moderna della Mini del 1959, prodotta dal 2001 sotto l'egida del costruttore tedesco BMW, e atta a ricoprire il segmento B. Indice 1 Genesi 1.1 Le concept E1 e ACV30 1.2 Il modello definitivo 2 Prima serie (R50, R52, R53, ...

Railway station in New Zealand Western HuttMetlink suburban railGeneral informationLocationHutt Road, Alicetown, Lower Hutt, New ZealandCoordinates41°12′43.22″S 174°53′23.48″E / 41.2120056°S 174.8898556°E / -41.2120056; 174.8898556Owned byBuilding is privately owned as a pub, platform owned by KiwiRailLine(s)Melling BranchPlatformsIsland (formerly)Tracks1ConstructionPlatform levels1Other informationFare zone4HistoryOpened14 April 1874Rebuilt1892, 1906Electr...

Alamgir IIKaisar MughalKaisar Mughal ke-14Berkuasa2 Juni 1754 – 29 November 1759PendahuluAhmad Shah BahadurPenerusShah Jahan IIIWaliImad-ul-Mulk (1754–1756)Najib-ul-Daula (1756–1759)Imad-ul-Mulk (1759)Informasi pribadiKelahiran(1699-06-06)6 Juni 1699Multan, Kekaisaran MughalKematian29 November 1759(1759-11-29) (umur 60)Kotla Fateh Shah, Kekaisaran MughalPemakamanMakam HumayunWangsaTimuriyahNama lengkapAziz-ud-din Alamgir IIAyahJahandar ShahIbuMuazzamabadi MahalPasanganZinat MahalFa...

American basketball player-coach Tony DelkDelk at the 2023 NBA Draft CombinePersonal informationBorn (1974-01-28) January 28, 1974 (age 50)Covington, Tennessee, U.S.Listed height6 ft 1 in (1.85 m)Listed weight189 lb (86 kg)Career informationHigh schoolHaywood (Brownsville, Tennessee)CollegeKentucky (1992–1996)NBA draft1996: 1st round, 16th overall pickSelected by the Charlotte HornetsPlaying career1996–2008PositionPoint guard / shooting guardNumber00, 28, 7, ...

2020 documentary film Class Action ParkDigital posterDirected by Seth Porges Chris Charles Scott III Produced by Michael Garber Chris M. Johnston Chris Lyon Seth Porges Narrated byJohn HodgmanCinematography Rob Senska Chris M. Johnston Edited by Chris Lyon Chris M. Johnston Music byThe Holladay BrothersProductioncompanies Pinball Party Productions Strategery Films Warner Max Distributed byHBO MaxRelease dates August 20, 2020 (2020-08-20) (Florida Film Festival)[1] A...

Helen Hull Jacobs Helen Jacobs nel 1933 Nazionalità Stati Uniti Tennis Termine carriera 1947 Hall of fame (1962) Carriera Singolare1 Vittorie/sconfitte Titoli vinti Miglior ranking 1º (1936) Risultati nei tornei del Grande Slam Australian Open - Roland Garros F (1930, 1934) Wimbledon V (1936) US Open V (1932, 1933, 1934, 1935) Doppio1 Vittorie/sconfitte Titoli vinti Miglior ranking Risultati nei tornei del Grande Slam Australian Open - Roland...

Canadian baseball player (born 1983) Baseball player Russell MartinMartin with the Los Angeles Dodgers in 2007CatcherBorn: (1983-02-15) February 15, 1983 (age 41)East York, Ontario, CanadaBatted: RightThrew: RightMLB debutMay 5, 2006, for the Los Angeles DodgersLast MLB appearanceSeptember 28, 2019, for the Los Angeles DodgersMLB statisticsBatting average.248Home runs191Runs batted in771 Teams Los Angeles Dodgers (2006–2010) New York Yankees (2011–2012) ...
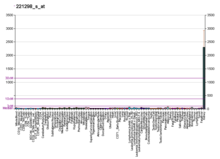
SLC22A8 المعرفات الأسماء المستعارة SLC22A8, OAT3, solute carrier family 22 member 8 معرفات خارجية الوراثة المندلية البشرية عبر الإنترنت 607581 MGI: MGI:1336187 HomoloGene: 20901 GeneCards: 9376 علم الوجود الجيني الوظيفة الجزيئية • sodium-independent organic anion transmembrane transporter activity• GO:0022891 transmembrane transporter activity• inorganic anion exchanger activity• ن�...

Questa voce sull'argomento stadi di calcio del Messico è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Stadio Jalisco Informazioni generaliStato Messico UbicazioneGuadalajara Inizio lavori30 novembre 1952 Inaugurazione31 gennaio 1960 Costo32 milioni di $ ProprietarioClubes Unidos de Jalisco, A.C ProgettoConstructora Jalisco S.A. de C.V.Constructora ARVA S.A. de C.V.,Jaime de Obeso, Javier Vallejo Informazioni tecnichePosti a sedere63 163 Struttur...

Japanese footballer Jungo Fujimoto 藤本 淳吾 Personal informationFull name Jungo FujimotoDate of birth (1984-03-24) 24 March 1984 (age 40)Place of birth Yamato, Kanagawa, JapanHeight 5 ft 8 in (1.73 m)Position(s) MidfielderYouth career1999–2001 Toko Gakuen H.S.College careerYears Team Apps (Gls)2002–2005 University of Tsukuba Senior career*Years Team Apps (Gls)2005–2010 Shimizu S-Pulse 139 (31)2011–2013 Nagoya Grampus 94 (14)2014–2015 Yokohama F. Marinos 45 (...

Chinese scholar In this Chinese name, the family name is Lu. Lu JiuyuanLu JiuyuanBorn1139Died1192 Lu Jiuyuan (Chinese: 陸九淵; pinyin: Lù Jiǔyuān; 1139–1192), or Lu Xiangshan (陸象山; Lù Xiàngshān), was a Chinese philosopher and writer who founded the school of the universal mind, the second most influential Neo-Confucian school. He was a contemporary and the main rival of Zhu Xi. In East Asia and the Western World, he is known by his honorific name rather than his priva...

مورينو توريتشيللي معلومات شخصية الميلاد 23 يناير 1970 (العمر 54 سنة)إربة [لغات أخرى] الطول 1.84 م (6 قدم 1⁄2 بوصة) مركز اللعب مدافع الجنسية إيطالي المسيرة الاحترافية1 سنوات فريق مشاركات (أهداف) (هـ.) 1990–1992 US Folgore Caratese ASD [الإنجليزية] 57 (3) 1992–1998 يوفنتوس 152 (1...

كاثرين الأولى، إمبراطورة اللاتينية (بالفرنسية: Catherine de Courtenay) معلومات شخصية تاريخ الميلاد 25 نوفمبر 1274 الوفاة 11 أكتوبر 1307 (32 سنة) باريس مكان الدفن كاتدرائية سان دوني مواطنة فرنسا الزوج شارل كونت فالوا (8 فبراير 1301–) الأولاد كاثرين الثانية، إمبراط...

Taman Pintar Yogyakarta Pintu gerbang Taman Pintar, 2015 Lokasi di Indonesia Informasi Lokasi Kota Yogyakarta, D.I. Yogyakarta Negara Indonesia Koordinat 7°48′00″S 110°21′54″E / 7.8°S 110.365°E / -7.8; 110.365 Pemilik Kesultanan Ngayogyakarta Hadiningrat, dikuasai oleh Pemerintah Kota Yogyakarta Jenis objek wisata Taman rekreasi dan edukasi Situs web http://tamanpintar.com/ Taman Pintar Yogyakarta (bahasa Jawa: ꦠꦩꦤ꧀ꦥꦶꦤ꧀ꦠꦂꦔ�...

German national public radio broadcaster This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Deutschlandradio – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this message) DeutschlandradioHeadquarters in CologneTypePublic-service sound broadcastingCountryGermanyBroadca...

Tyrell Malacia Malacia bersama Feyenoord pada tahun 2019Informasi pribadiNama lengkap Tyrell Johannes Chicco Malacia[1]Tanggal lahir 17 Agustus 1999 (umur 24)[2]Tempat lahir Rotterdam, BelandaTinggi 1,70 m (5 ft 7 in)[2]Posisi bermain Bek kiriInformasi klubKlub saat ini Manchester UnitedNomor 12Karier junior2008–2017 FeyenoordKarier senior*Tahun Tim Tampil (Gol)2017–2022 Feyenoord 98 (4)2022– Manchester United 22 (0)Tim nasional‡2014–2015...

Grand Prix Sepeda Motor F.I.M. musim 2022 Sebelum: 2021 Sesudah: 2023 MotoGP musim 2022Moto2 musim 2022Moto3 musim 2022Juara Dunia Dominique Aegerter Dominique Aegerter adalah Pemenang Piala Dunia MotoE 2022 MotoE musim 2022 (dikenal secara resmi sebagai FIM Enel MotoE World Cup 2022 karena alasan sponsor) akan menjadi musim keempat MotoE untuk balap motor listrik, dan merupakan bagian dari musim Grand Prix Sepeda Motor F.I.M. ke-74. Ini akan menjadi musim terakhir Energica menjadi pemasok t...

Football league seasonChinese Super LeagueSeason2019Dates1 March – 1 December 2019ChampionsGuangzhou Evergrande Taobao (8th title)RelegatedBeijing RenheAFC Champions LeagueGuangzhou Evergrande TaobaoBeijing Sinobo GuoanShanghai SIPGShanghai Greenland ShenhuaMatches played240Goals scored741 (3.09 per match)Top goalscorerEran Zahavi (29 goals)Biggest home winShanghai Greenland Shenhua 5–1 Beijing Renhe(7 April 2019)Jiangsu Suning 5–1 Guangzhou R&F(21 April 2019)Shanghai SIPG 5–...

关于与「王刚 (全国政协前副主席)」標題相近或相同的条目页,請見「王刚」。 王刚中国人民政治协商会议第十一届全国委员会副主席任期2008年3月—2013年3月 个人资料出生1942年10月(81歲) 滿洲國吉林省扶余县籍贯吉林扶余国籍 中华人民共和国政党 中国共产党 学历 吉林大学哲学系 王刚(1942年10月5日—),男,汉族,吉林扶余人,中华人民共和国政�...

Questa voce sull'argomento stagioni delle società calcistiche francesi è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Voce principale: Olympique de Marseille. Olympique de MarseilleStagione 1994-1995Sport calcio Squadra Olympique Marsiglia Allenatore Marc Bourrier, poi Gérard Gili, poi Henri Stambouli Presidente Bernard Tapie, poi Pierre Cangioni, poi Bernard Caïazzo, poi Jean-Michel Ripa Division 21º Coppa di FranciaSemifinale Coupe de la L...
