Lixisenatide
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Dianne Reeves dalam acara Boston Pops, 1 Juni 2007 Dianne Reeves (lahir 23 Oktober 1956) adalah penyanyi jazz asal Amerika Serikat, terkenal karena kemampuan menyanyi panggungnya yang sama baiknya dengan yang ada di albumnya. Dia adalah salah satu penyanyi penting dalam dunia jazz. Saat ini dia tinggal di Denver, Colorado, Amerika Serikat. Pranala luar (Inggris) Situs web resmi (Inggris) (Prancis) Interview Video Dianne Reeves di Bamboo-music.com - English & French versions Diarsipkan 201...

Michael Schulze Informasi pribadiNama lengkap Michael SchulzeTanggal lahir 13 Januari 1989 (umur 35)Tempat lahir Göttingen, Jerman BaratTinggi 1,85 m (6 ft 1 in)Posisi bermain Bek kananInformasi klubKlub saat ini Energie Cottbus (pinjaman dari VfL Wolfsburg)Karier junior0000–2008 SSV Vorsfelde2001–2008 VfL WolfsburgKarier senior*Tahun Tim Tampil (Gol)2008–kini VfL Wolfsburg II 113 (7)2011–kini VfL Wolfsburg 2 (0)2013 → Energie Cottbus (pinjaman) 8 (2) * Penampi...
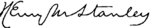
Sir Henry Morton StanleyStanley pada tahun 1872LahirJohn Rowlands(1841-01-28)28 Januari 1841Denbigh, Wales, UKMeninggal10 Mei 1904(1904-05-10) (umur 63)London, Inggris, UKPenghargaanVega Medal (1883)Tanda tangan Henry Morton Stanley, 1890 Sir Henry Morton Stanley GCB (lahir John Rowlands; 28 Januari 1841 – 10 Mei 1904) adalah wartawan dan penjelajah Wales-Amerika[1][2] yang terkenal untuk eksplorasi Afrika tengah dan pencariannya untuk misionaris dan penjelajah David L...

Ed Perlmutter Edwin George Perlmutter (lahir 1 Mei 1953) adalah seorang politikus Amerika Serikat yang menjabat sebagai anggota DPR sejak 2007. Ia adalah anggota Partai Demokrat. Ia sebelumnya menjabat sebagai Senator Negara Bagian Colorado dari 1995 sampai 2003. Pranala luar Wikimedia Commons memiliki media mengenai Ed Perlmutter. Wikisumber memiliki karya asli dari atau mengenai: Ed Perlmutter U.S. Congressman Ed Perlmutter Diarsipkan 2007-07-25 di Wayback Machine. official U.S. House websi...

JambalayaJambalaya dengan ayam, sosis, beras, tomat, seledri, dan rempah-rempah.JenisStewBahan utamaDaging, sayuran, kaldu, nasiSunting kotak info • L • BBantuan penggunaan templat ini Media: Jambalaya Video ini menunjukkan perbedaan antara Creole dan Cajun Jambalaya. Jambalaya dibuat berbeda tergantung pada daerahnya dan terinspirasi oleh budaya Prancis dan Spanyol. Jambalaya (/[invalid input: 'icon']ˌdʒʌmbəˈlaɪ.ə/ JUM-bə-LY-ə) adalah makanan yang berasal dar...

City in California, United States City in California, United StatesSutter CreekCityA view of Main Street (Old Highway 49) in Sutter Creek.Nickname: Jewel of the gold countryLocation in Amador CountySutter CreekLocation in CaliforniaShow map of CaliforniaSutter CreekSutter Creek (the United States)Show map of the United StatesCoordinates: 38°23′35″N 120°48′09″W / 38.39306°N 120.80250°W / 38.39306; -120.80250Country United StatesState Californi...

This article is about the year 509. For other uses, see 509 (disambiguation). This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: 509 – news · newspapers · books · scholar · JSTOR (October 2017) (Learn how and when to remove this template message) Calendar year Millennium: 1st millennium Centuries: 5th century 6th ...

Italian restaurant in New York City Al CoroAl Coro in December 2023Restaurant informationHead chefMelissa RodriguezChefKatherine RockPastry chefGeorgia WodderFood typeItalian cuisineRating (Michelin Guide)Street address85 Tenth AvenueCityNew York CityStateNew YorkPostal/ZIP Code10011CountryUnited StatesCoordinates40°44′36.3″N 74°0′27.2″W / 40.743417°N 74.007556°W / 40.743417; -74.007556Websitewww.alcoro-nyc.com Al Coro is a restaurant in New York City, New ...

موسيقى تركيةمعلومات عامةالبلد تركيا أصول الأسلوب موسيقى الشرق الأوسط تعديل - تعديل مصدري - تعديل ويكي بيانات جزء من سلسلة حول ثقافة تركيا الفلكلور والأساطير الأساطير الفلكلور الدين المسيحية الإسلام الفنون رقص العمارة الأدب شعر الموسيقى والمسرح موسيقى مسرح الإعلام تل�...

1989 television film directed by Lucio Fulci The Sweet House of HorrorsDVD cover for The Sweet House of HorrorsItalianLa dolce casa degli orrori[1] GenreHorror[2]Screenplay by Vincenzo Mannino Gigliola Battaglini[3] Story byLucio Fulci[3]Directed byLucio Fulci[3]Starring Jean-Christophe Brétigniere Cinzia Monreale Ilary Blasi Lubka Cibulova Composer Vince Tempera[3] ProductionProducers Massimo Manasse Marco Grillo Spina[1] Cinematograph...

Durban iTheku (Zulu) Clockwise from top left: Durban CBD, Ushaka Marine World, Suncoast Casino and Entertainment World, Moses Mabhida Stadium, Inkosi Albert Luthuli International Convention Centre and Durban City Hall. BenderaLambang kebesaranCountrySouth AfricaProvince[[]]District[[Invalid Data-key599054 District Municipality|Invalid Data-key599054]]Municipality[[Invalid Data-key599054 Local Municipality |Invalid Data-key599054]]Luas[1] • KotaInvalid Data-key599.054 ...

Football tournament season 2018–19 Cupa RomânieiCupa României 2018–19Tournament detailsCountry RomaniaTeams133Defending championsUniversitatea CraiovaFinal positionsChampionsViitorul ConstanțaRunner-upAstra Giurgiu← 2017–182019–20 → The 2018–19 Cupa României was the 81st season of the annual Romanian primary football knockout tournament. As winners, Viitorul Constanța, qualified for the second qualifying round of the 2019–20 UEFA Europa League. Time...

State Public Historical Library of RussiaГосударственная публичная историческая библиотека РоссииState Historic Public Library of Russia Main Hall of Historical building55°45′22″N 37°38′24″E / 55.7561°N 37.6401°E / 55.7561; 37.6401LocationMoscow, RussiaTypeHistorical libraryEstablished1938Other informationWebsitehttp://www.shpl.ru/ The State Public Historical Library of Russia was founded in 1938 under the...

Brazilian footballer and manager This article is about the Brazilian football manager and former left-back. For similar uses, see Silvinho. In this Portuguese name, the first or maternal family name is Mendes and the second or paternal family name is Campos. Sylvinho Sylvinho in 2019Personal informationFull name Sylvio Mendes Campos JúniorDate of birth (1974-04-12) 12 April 1974 (age 50)Place of birth São Paulo, BrazilHeight 1.72 m (5 ft 8 in)Position(s) Left backTea...

Culinary cream sauce Peppercorn SauceSteak au poivre with a peppercorn sauceTypeCreamCourseAnyPlace of originFranceServing temperatureHotMain ingredientsPeppercorns and heavy creamIngredients generally usedButter, wine, shallots, brandy or cognac and additional seasoningsVariationsWhiskey substituted for brandy Media: Peppercorn Sauce Peppercorn sauce is a culinary cream sauce prepared with peppercorn, which is prepared as a reduction of the cream in the cooking process.[1&...

La Chapelle-BayvelcomuneLa Chapelle-Bayvel – Veduta LocalizzazioneStato Francia Regione Normandia Dipartimento Eure ArrondissementBernay CantoneBeuzeville TerritorioCoordinate49°16′N 0°24′E / 49.266667°N 0.4°E49.266667; 0.4 (La Chapelle-Bayvel)Coordinate: 49°16′N 0°24′E / 49.266667°N 0.4°E49.266667; 0.4 (La Chapelle-Bayvel) Superficie4,85 km² Abitanti321[1] (2009) Densità66,19 ab./km² Altre informazioniCod. po...

この項目には、一部のコンピュータや閲覧ソフトで表示できない文字が含まれています(詳細)。 数字の大字(だいじ)は、漢数字の一種。通常用いる単純な字形の漢数字(小字)の代わりに同じ音の別の漢字を用いるものである。 概要 壱万円日本銀行券(「壱」が大字) 弐千円日本銀行券(「弐」が大字) 漢数字には「一」「二」「三」と続く小字と、「壱」「�...

提示:此条目页的主题不是中國—瑞士關係。 關於中華民國與「瑞」字國家的外交關係,詳見中瑞關係 (消歧義)。 中華民國—瑞士關係 中華民國 瑞士 代表機構駐瑞士台北文化經濟代表團瑞士商務辦事處代表代表 黃偉峰 大使[註 1][4]處長 陶方婭[5]Mrs. Claudia Fontana Tobiassen 中華民國—瑞士關係(德語:Schweizerische–republik china Beziehungen、法�...

يفتقر محتوى هذه المقالة إلى الاستشهاد بمصادر. فضلاً، ساهم في تطوير هذه المقالة من خلال إضافة مصادر موثوق بها. أي معلومات غير موثقة يمكن التشكيك بها وإزالتها. (ديسمبر 2018) هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها...

Lake in Belknap County, New Hampshire Squam LakeView from the cliffs of East RattlesnakeSquam LakeLocationGrafton County, Carroll County, and Belknap County, New HampshireCoordinates43°44′43″N 71°31′34″W / 43.74528°N 71.52611°W / 43.74528; -71.52611Primary outflowsSquam RiverBasin countriesUnited StatesMax. length7.0 mi (11.3 km)Max. width4.6 mi (7.4 km)Surface area6,791 acres (2,748 ha)Max. depth99 ft (30 m)Surface e...