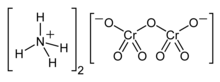
Ammonium dichromate
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

الشيخ ياسر العواضي مناصب عضو مجلس النواب اليمني[1] في المنصب27 أبريل 2003 – 15 نوفمبر 2021 معلومات شخصية الميلاد 1 مارس 1978 عزلة ردمان آل عوض الوفاة 15 نوفمبر 2021 (43 سنة) [2] القاهرة[2] مواطنة اليمن الديانة الإسلام الأب أحمد سالم أحمد عبد الله ا...

Questa voce sull'argomento perciformes è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Come leggere il tassoboxDentex Dentex dentex Classificazione scientifica Dominio Eukaryota Regno Animalia Sottoregno Eumetazoa Phylum Chordata Subphylum Vertebrata Superclasse Gnathostomata Classe Actinopterygii Sottoclasse Osteichthyes Superordine Acanthopterygii Ordine Perciformes Sottordine Percoidei Famiglia Spar...

Free and open-source cross-platform widget toolkit for creating graphical user interfaces For other uses, see GTK (disambiguation). GTK version 4 (gtk4-widget-factory, a collection of examples that demonstrate many of the GUI widgets)Original author(s)Spencer Kimball, Peter MattisDeveloper(s)The GNOME Project, eXperimental Computing Facility (XCF)Initial releaseApril 14, 1998; 25 years ago (1998-04-14)Stable release4.14.1 / March 16, 2024; 24 days ago (2024...

Village Development Committee in Kosi Zone, NepalGogane गोगनेVillage Development CommitteeCountry NepalZoneKosi ZoneDistrictBhojpur DistrictPopulation (1991) • Total2,283Time zoneUTC+5:45 (Nepal Time) Gogane is a Village Development Committee in Bhojpur District in the Kosi Zone of eastern Nepal. At the time of the 1991 Nepal census it had a population of 2283 persons residing in 415 individual households.[1] References ^ Nepal Census 2001. ...

Stadio Alfredo VivianiCampo Sportivo ItaliaCampo Sportivo del Littorio Informazioni generaliStato Italia UbicazioneViale Guglielmo Marconi, 21985100 Potenza Inizio lavori1934 Inaugurazione1934 Ristrutturazione2007-2008, 2016-2017, 2018-2019, 2021-2022 ProprietarioDemanio dello Stato (in locazione al Comune di Potenza) GestoreComune di Potenza Prog. strutturaleGennaro Laurini Informazioni tecnichePosti a sedere4 977[1][2] Strutturastile aperto Copertura1.574 posti (tr...

Questa voce sull'argomento attori statunitensi è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Julie MeadowsDati biograficiNazionalità Stati Uniti Dati fisiciAltezza168 cm Peso52 kg Etniacaucasico Occhicastani Capellibiondi Misure34C-26-34 Dati professionaliFilm girati299[1] Sito ufficiale Modifica dati su Wikidata · Manuale Julie Meadows (Texarkana, 3 febbraio 1974) è un'ex at...

この記事は検証可能な参考文献や出典が全く示されていないか、不十分です。出典を追加して記事の信頼性向上にご協力ください。(このテンプレートの使い方)出典検索?: コルク – ニュース · 書籍 · スカラー · CiNii · J-STAGE · NDL · dlib.jp · ジャパンサーチ · TWL(2017年4月) コルクを打ち抜いて作った瓶の栓 コルク(木栓、�...

Canadian-based gold and silver mining company Kinross Gold CorporationCompany typePublicTraded asTSX: KNYSE: KGCIndustryMetals and MiningFounded1993FounderRobert BuchanHeadquartersToronto, OntarioKey peopleJ. Paul Rollinson, CEO [1]ProductsGold, SilverRevenueUS$3.279 billion (2021)[2]Operating incomeUS$464 million (2021)Net incomeUS$221 million (2021)Websitewww.kinross.com Kinross Gold Corporation is a Canadian-based gold and silver mining company founded in 1993 and...

Gonzalo Colsa Albendea Nazionalità Spagna Altezza 185 cm Calcio Ruolo Centrocampista Termine carriera 2013 CarrieraGiovanili 1996-1998 Racing SantanderSquadre di club1 1997-2001 Racing Santander39 (3)1999→ CD Logroñés5 (0)2001-2002 Atlético Madrid18 (0)2002-2003 Real Valladolid37 (5)2003-2004 Maiorca32 (3)2004-2006 Atlético Madrid44 (4)2006-2012 Racing Santander188 (14)2012-2013 Mirandés3 (0)Nazionale 1999 Spagna U-20? (?)Palmarès ...

Community college in Petoskey, Michigan, U.S. This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: North Central Michigan College – news · newspapers · books · scholar · JSTOR (April 2015) (Learn how and when to remove this message) North Central Michigan CollegeTypePublic community collegeEstablished1958Presiden...

Japanese television series An evil version of Minilla battles the Toho superhero Greenman. Go! Greenman (行け! グリーンマン, Ike! Greenman) is a tokusatsu television series Kyodai Hero kaiju produced by Toho in 1973. It ran from November 12, 1973, to September 27, 1974. It emerged as a follow-up series to Ike! Godman, but the two share no continuity. Compared to the anthology-like storylines of Ike! Godman, Go! Greenman has a single overarching plot. Synopsis Deep beneath Japan, a dem...

Pour les articles homonymes, voir Marino. Giambattista MarinoBiographieNaissance 14 octobre 1569Naples, Royaume de NaplesDécès 25 mars 1625 (à 55 ans)Naples, Royaume de NaplesActivités Poète, écrivain, dramaturgePériode d'activité 1590-1625Autres informationsMembre de Accademia degli UmoristiAccademia degli OziosiMouvement BaroqueGenres artistiques Poésie narrative, canzone, sonnet, madrigalŒuvres principales L'Adone (d), La strage degli innocenti (d)modifier - mod...

此条目序言章节没有充分总结全文内容要点。 (2019年3月21日)请考虑扩充序言,清晰概述条目所有重點。请在条目的讨论页讨论此问题。 哈萨克斯坦總統哈薩克總統旗現任Қасым-Жомарт Кемелұлы Тоқаев卡瑟姆若马尔特·托卡耶夫自2019年3月20日在任任期7年首任努尔苏丹·纳扎尔巴耶夫设立1990年4月24日(哈薩克蘇維埃社會主義共和國總統) 哈萨克斯坦 哈萨克斯坦政府...

Piers MorganMorgan di acara PaleyFest 2013LahirPiers Stefan O'Meara30 Maret 1965 (umur 59)Newick, Sussex, Inggris[1]KebangsaanBritaniaPendidikanSekolah ChaileyAlmamaterHarlow CollegePekerjaanPresenter televisi, penulis, jurnalisTahun aktif1985–sekarangTempat kerjaSouth London News (1985–88)The Sun (1989–94)News of the World (1994–95)Daily Mirror (1995–2004)Dikenal atasPenyunting koranTuan rumah acara televisiTelevisiBritain's Got TalentAmerica's Got TalentThe Cele...
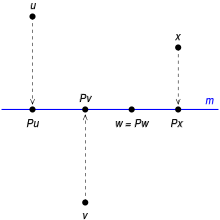
Disambiguazione – Se stai cercando il metodo di rappresentazione grafica, vedi Proiezioni ortogonali. La proiezione ortogonale di un cubo su un piano verticale. In algebra lineare e analisi funzionale, una proiezione è una trasformazione lineare P {\displaystyle P} definita da uno spazio vettoriale in sé stesso (endomorfismo) che è idempotente, cioè tale per cui P 2 = P {\displaystyle P^{2}=P} : applicare due volte la trasformazione fornisce lo stesso risultato che applicandola una vol...

Champ Car CategoriaMonoposto NazioneMondiale Prima edizione1979 (sotto il nome CART) Ultima edizione2007 Piloti18 Squadre9 CostruttoriPanoz MotoriFord Cosworth PneumaticiB Pilota campione (2007) Sébastien Bourdais Squadra campione (2007) Panoz Sito web ufficialewww.champcarworldseries.com/ Noteconfluita nel 2008 nella IRL per formare la IndyCar Series Champ Car Champ Car era il nome della serie automobilistica precedentemente nota come CART (acronimo di Championship Auto Racing Teams) e...

This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Longing for You EP – news · newspapers · books · scholar · JSTOR (April 2016) (Learn how and when to remove this message) 1985 EP by CacumenLonging for YouEP by CacumenReleased1985GenreHard rockLength17:33LabelBoom RecordsProducerErnest KrichelCacumen chronology Bad...

在羅馬的圣安当院长堂 系列条目天主教會自权個別教會 拉丁礼教会使用拉丁十字,拜占庭礼教会使用宗主十字。 根据教礼排列: 拉丁礼 拉丁礼 拜占庭或希腊礼 阿尔巴尼亚 白俄罗斯 保加利亚 克罗地亚和塞尔维亚(英语:Byzantine Catholic Church of Croatia and Serbia) 希腊 匈牙利 意大利-阿尔巴尼亚 马其顿 默基特 罗马尼亚(英语:Romanian Greek-Catholic Church) 俄罗斯 鲁塞尼亚 斯洛�...

Emergency protocol that prevents people or information from leaving an area This article is about the emergency measure. For the mass quarantine measure in a disease outbreak, see Stay-at-home order and COVID-19 lockdowns. For the practice of isolating prisoners, see Solitary confinement. For other uses, see Lockdown (disambiguation). A lockdown (/ˈlɒkˌdaʊn/ ⓘ) is a restriction policy for people, community or a country to stay where they are, usually due to specific risks that could...

Pour l’article homonyme, voir Archaea (genre). Archaea Halobacterium sp. souche NRC-1.Chaque cellule mesure environ 5 μm de long.Classification DomaineArchaeaWoese, Kandler (en) & Wheelis (en), 1990 RègneArchaeaWoese, Kandler (en) & Wheelis (en), 1990[1] Synonymes Archaebacteria Mendosicutes Metabacteria Classification phylogénétique Position : Domaine : Bacteria Domaine : Archaea Règne : Archaea Phylum : Crenarchaeota Classe&#...