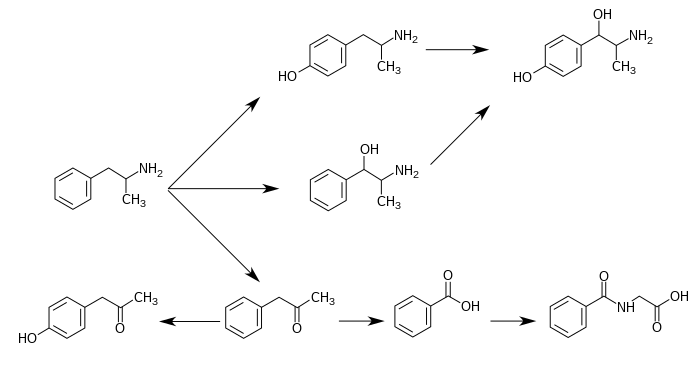
4-Hydroxyamphetamine
| ||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Phi2 Orionis Classificazionegigante arancione Classe spettraleK0IIIb C ~ Distanza dal Sole116 anni luce CostellazioneOrione Redshift98,72 ± 0,90 Coordinate(all'epoca J2000.0) Ascensione retta05h 36m 54,3879s Declinazione+09° 17′ 26,422″ Lat. galattica-11,9471° Long. galattica195,8449° Dati fisiciRaggio medio7,59 R⊙ Massa1,61 M⊙ Acceleraz. di gravità in superficie2,89 logg Temperaturasuperficiale4897,79 K (media) Metallicità32% del Sole Dati os...

Hotel in Fort Lauderdale, United States Seminole Hard Rock Hotel & Casino(June 2021) Address 1 Seminole WayHollywood, Broward County, Florida 33314Opening dateMay 1, 2004[1]ThemeRock and Roll / MusicNo. of rooms1,275Total gaming space140,000 sq ft (13,000 m2)Notable restaurantsHard Rock CafeCouncil Oak Steaks & SeafoodKuroCasino typeLand-basedOwnerSeminole Gaming(Seminole Tribe of Florida)Websitewww.seminolehardrockhollywood.com Seminole Hard Rock Hotel & Ca...

Terra Formarsテラフォーマーズ(Tera Fōmāzu)GenreAdventure[1]Horror[2]fiksi ilmiah[1][2] MangaPengarangYū SasugaIlustratorKenichi TachibanaPenerbitShueishaPenerbit bahasa InggrisNA Viz MediaImprintYoung Jump ComicsMajalahMiracle Jump (January 13 – December 13, 2011)Weekly Young Jump (April 26, 2012 – present)DemografiSeinenTerbit13 Januari, 2011 – sekarangVolume22 Informasi tambahan MangaTerra for PolicePengarangManpuku DouIlustratorKaito Shibano...

Article principal : Configuration d'aile. Le Curtiss P-40 Warhawk, monoplan à ailes basses. Un monoplan est un aéronef n'ayant qu'une seule paire d'ailes comme plan de sustentation[1]. Depuis la fin des années 1930, la plupart des aéronefs adoptent cette configuration, alors que les multi-plans étaient jusqu'alors privilégiées. L'amélioration constante et l'augmentation de puissance des motorisations ont en effet permis de compenser la diminution de portance liée à la diminutio...

Questa voce sull'argomento calciatori spagnoli è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Borja Pérez Nazionalità Spagna Altezza 183 cm Calcio Ruolo Attaccante Termine carriera 2016 Carriera Giovanili Real Madrid Santa Maria Leganés Squadre di club1 2002-2003 Leganés B? (?)2003-2005 Leganés71 (18)2005-2006 Real Valladolid B35 (12)2006-2009 Alicante74 (21)2...

Portugalau Concours Eurovision 2022 Données clés Pays Portugal Chanson Saudade, saudade Interprète MARO Langue Portugais, anglais Sélection nationale Radiodiffuseur RTP Type de sélection Festival da Canção 2022 Date 12 mars 2022 Concours Eurovision de la chanson 2022 Position en demi-finale 4e (208 points, qualifiée) Position en finale 9e (207 points) 2021 2023 modifier Le Portugal est l'un des quarante pays participants du Concours Eurovision de la chanson 2022, qui ...

Genus of birds Pteroptochos Moustached turca (Pteroptochos megapodius) Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Chordata Class: Aves Order: Passeriformes Family: Rhinocryptidae Genus: PteroptochosKittlitz, 1830 Type species Pteroptochos megapodiusvon Kittlitz, 1830 Pteroptochos is a genus of birds in the tapaculo family Rhinocryptidae.[1] Species The genus contains the following three species:[2] Genus Pteroptochos – Kittlitz,, 1830 – three spe...

Fédération de football d'Asie du Sud-Est Sigle AFF Sport(s) représenté(s) Football Création 31 janvier 1984 Siège Malaisie, Petaling Jaya, Selangor Nations membres 12 membres Site internet (en) Site officiel modifier La Fédération de football d'Asie du Sud-Est ou Fédération de football de l'ASEAN (en anglais ASEAN football federation, couramment désignée par le sigle AFF) est une fédération internationale de football regroupant les fédérations nationales membres de ...

此條目可参照英語維基百科相應條目来扩充。 (2017年8月)若您熟悉来源语言和主题,请协助参考外语维基百科扩充条目。请勿直接提交机械翻译,也不要翻译不可靠、低品质内容。依版权协议,译文需在编辑摘要注明来源,或于讨论页顶部标记{{Translated page}}标签。 密西西比州 美國联邦州State of Mississippi 州旗州徽綽號:木蘭之州地图中高亮部分为密西西比州坐标:30°13'N�...

此條目可参照英語維基百科相應條目来扩充。 (2021年5月6日)若您熟悉来源语言和主题,请协助参考外语维基百科扩充条目。请勿直接提交机械翻译,也不要翻译不可靠、低品质内容。依版权协议,译文需在编辑摘要注明来源,或于讨论页顶部标记{{Translated page}}标签。 约翰斯顿环礁Kalama Atoll 美國本土外小島嶼 Johnston Atoll 旗幟颂歌:《星條旗》The Star-Spangled Banner約翰斯頓環礁�...

انقلاب 1953 في إيران جزء من أزمة عبادان، والحرب الباردة، وتورط الولايات المتحدة في تغيير النظام التاريخ وسيط property غير متوفر. نهاية 19 أغسطس 1953 البلد إيران الموقع طهران تعديل مصدري - تعديل 32°25′40″N 53°41′17″E / 32.427908°N 53.688046°E / 32.427908; 53.688046 انقلا�...
Daily UK events related to the pandemic in 2022 United Kingdom COVID-19 timeline for July–December 2022 Previous period Next period Graph of COVID-19 cases for July–December 2022 from government data Timelines for other periods 2020 Jan-Jun, Jul-Dec 2021 Jan-Jun, Jul-Dec 2022 Jan-Jun, Jul-Dec 2023 2024 Regions during July–December 2022 England Northern Ireland Scotland Wales History in UK Government Response COVID-19 in UK Worldwide timeline List of UK timelinesvte The following is a ti...

US Air Force unit 2nd Bombardment Wing redirects here. For the 2nd Bombardment Wing of 1919–1945, see 2nd Bombardment Wing (World War II). This article needs to be updated. Please help update this article to reflect recent events or newly available information. (November 2022) 2nd Bomb WingBoeing B-52H bomber at Barksdale Air Force BaseFounded15 October 1947Country United StatesBranch United States Air ForceRoleBomberPart ofAir Force Global Strike CommandEighth Air ForceGarri...
Kolom Winogradsky awalnya Kolom Winogradsky setelah beberapa minggu Kolom Winogradsky adalah suatu miniatur ekosistem buatan untuk membiakkan mikrob yang menyerupai kondisi ekologis sebenarnya dengan menyediakan sumber bakteri jangka panjang untuk pengkayaan kultur.[1] Kolom Winogradsky adalah salah satu cara sederhana untuk mempelajari hubungan silang antara dua komponen suatu lingkungan alami di laboratorium.[1] Penemuan Kolom Winogradsky merupakan ide seorang ilmuwan Rusia ...

Artikel ini membutuhkan rujukan tambahan agar kualitasnya dapat dipastikan. Mohon bantu kami mengembangkan artikel ini dengan cara menambahkan rujukan ke sumber tepercaya. Pernyataan tak bersumber bisa saja dipertentangkan dan dihapus.Cari sumber: Lingkungan dan bangunan pertanian – berita · surat kabar · buku · cendekiawan · JSTOR (Maret 2014) Lingkungan dan bangunan pertanian (LBP) adalah salah satu cabang disiplin ilmu dalam teknik pertanian yang fo...

Mate tea served in traditional gourd cups in Argentina. A cup of freshly made mate. The Argentine tea culture is influenced by local and imported varieties and customs. The country is a major producer of tea (Camellia sinensis), but is best known for the cultivation and consumption of mate, made with the leaves of the local yerba mate plant. History When Jesuit missionaries first came to Argentina, they tried to ban the popular indigenous tea, yerba mate, out of concern about its addictive qu...

Cet article est une ébauche concernant l’art et une chronologie ou une date. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Chronologies Données clés 1656 1657 1658 1659 1660 1661 1662Décennies :1620 1630 1640 1650 1660 1670 1680Siècles :XVe XVIe XVIIe XVIIIe XIXeMillénaires :-Ier Ier IIe IIIe Chronologies thématiques Art Architecture, Arts...

ARIA Chartsnumber-one singles of 2009< 20082010 >Other Australian number-one charts of 2009albumsurban singlesdance singlesclub tracksdigital tracksTop Australian singles and albums of 2009Triple J Hottest 100top 25 singlestop 25 albums The Black Eyed Peas achieved three number-one singles in 2009 for Boom Boom Pow, I Gotta Feeling and Meet Me Halfway, spanning 14 weeks in total at number-one on the chart. The ARIA Singles Chart ranks the best-performing singles in Australia. Its data,...

Decorative technique Stucco in the Court of the Lions of the Alhambra (14th century), in Granada, Spain. Arabesques are mixed here with calligraphic motifs and muqarnas sculpting. Stucco decoration in Islamic architecture refers to carved or molded stucco and plaster. The terms stucco and plaster are used almost interchangeably in this context to denote most types of stucco or plaster decoration with slightly varying compositions.[1] This decoration was mainly used to cover walls and ...

Slovenský Pohár 2020-2021Slovnaft Cup 2020-2021 Competizione Slovenský Pohár Sport Calcio Edizione 28ª Date dal 25 luglio 2020al 19 maggio 2021 Luogo Slovacchia Partecipanti 229 Risultati Vincitore Slovan Bratislava(10º titolo) Secondo Žilina Statistiche Incontri disputati 216 Gol segnati 842 (3,9 per incontro) Cronologia della competizione 2019-2020 2021-2022 Manuale La Coppa di Slovacchia 2020-2021, conosciuta anche come Slovnaft Cup per ragioni di sponsorizzazion...