Vosaroxin
| |||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Stasiun Gumma-Sōja群馬総社駅Stasiun Gumma-Sōja pada Maret 2006LokasiSōja-machi Ueno 588, Maebashi, Gunma(群馬県前橋市総社町植野588)JepangOperatorJR EastJalurJalur JōetsuSejarahDibuka1921PenumpangFY20131.534 per hari Operasi layanan Lua error in Modul:Adjacent_stations at line 221: Jalur tidak dikenal "Agatsuma". Sunting kotak info • L • BBantuan penggunaan templat ini Stasiun Gumma-Sōja (群馬総社駅code: ja is deprecated , Gumma-Sōja-eki) ...

Situ Rawa Gede Jonggol atau Situ Rawa Gede Sukamakmur, Jonggol adalah sebuah danau (Sd., situ atau setu berarti telaga, gede berarti besar) yang terletak di desa Sirnajaya, Kecamatan Sukamakmur, Kabupaten Bogor. Situ Rawa Gede memiliki luas sekitar 4,6 hektar dan terletak berada di ketinggian + 1072 m dpl ini menjadi tempat wisata yang cukup eksotis di wilayah Bogor Timur. Akses Objek wisata ini bisa dijangkau oleh motor maupun mobil. Jaraknya sekitar dua jam dari Jakarta dengan mobil dengan ...

Canton de Cambrai-Est Situation du canton de Cambrai-Est dans le département de Nord. Administration Pays France Région Nord-Pas-de-Calais Département Nord Arrondissement(s) Cambrai Chef-lieu Cambrai Conseiller général Mandat Nicolas Siegler 2011-2015 Code canton 59 13 Démographie Population 23 414 hab. (2012) Densité 304 hab./km2 Géographie Coordonnées 50° 11′ 42″ nord, 3° 17′ 05″ est Superficie 77 km2 Subdivisions Commune...

2004 United States House of Representatives elections in Minnesota ← 2002 November 2, 2004 (2004-11-02) 2006 → All 8 Minnesota seats to the United States House of Representatives Majority party Minority party Party Democratic (DFL) Republican Last election 4 seats, 49.87% 4 seats, 46.76% Seats before 4 4 Seats won 4 4 Seat change Popular vote 1,399,624 1,236,094 Percentage 51.42% 45.42% Swing 1.55% 1.34% Elections in...

Carlos Queiroz Informasi pribadiNama lengkap Carlos Manuel Brito Leal QueirozTanggal lahir 1 Maret 1953 (umur 71)Tempat lahir Nampula, Portuguese MozambiquePosisi bermain Penjaga gawang[1]Informasi klubKlub saat ini Kolombia (manajer)Karier juniorTahun Tim1974–1978 Ferroviário de NampulaKepelatihanTahun Tim 1989–1991 Portugal U-201991–1993 Portugal1994–1996 Sporting CP1996 NY/NJ MetroStars1996–1997 Nagoya Grampus Eight1998–1999 Uni Emirat Arab2000–2002 Afrika Sela...

Samut Songkhram สมุทรสงครามProvinsi BenderaJulukan: Mae KlongPeta Thailand memperlihatkan Provinsi Samut SongkhramNegaraThailandIbukotaSamut SongkhramPemerintahan • GubernurKhanchat Tansathian (sejak Oktober 2016)Luas • Total416,7 km2 (1,609 sq mi)Peringkatke-76Populasi (2017) • Total193.902 • Peringkatke-75 • Kepadatan0,047/km2 (0,12/sq mi) • Peringkat kepadatanke-...

British midwife who specializes in the study of female genital mutilation Comfort MomohMBEby Julie GoughBornc. 1962[1]Lagos, Nigeria[2]EducationBSc (2002), women's healthcare, Florence Nightingale School of Nursing and Midwifery, King's College London.[3]OccupationMidwifeKnown forWorking with FGM survivorsSpouseRobert Momoh[2]ChildrenTwo[2]Awards MBE (2008) Honorary doctorate, Middlesex University (2008) Fellowship, Royal College of Midwives (...

City and capital of North Maluku, Indonesia Town in Maluku, IndonesiaSofifiTownOpenStreetMapSofifiLocation of the town in HalmaheraCoordinates: 0°43′28″N 127°34′50″E / 0.72444°N 127.58056°E / 0.72444; 127.58056Country IndonesiaRegionMalukuProvince North MalukuArea • Total35.0 km2 (13.5 sq mi)Elevation15 m (49 ft)Population (2020 Census) • Total2,498 [1]Time zoneUTC+9 (Indonesia Eas...

Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. Le ton de cet article ou de cette section est trop lyrique ou dithyrambique (mars 2019). Modifiez l'article pour adopter un ton neutre et encyclopédique (c’est à dire ?) ou discutez-en. Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. La traduction de cet article ou de cette section doit être revue (avril 2016). Le contenu est difficilement compréhensible vu les erreurs ...

American college basketball season 2010–11 Notre Dame Fighting Irish men's basketballOld Spice Classic ChampionsNCAA Tournament, Round of 32ConferenceBig East ConferenceRankingCoachesNo. 14APNo. 5Record27–7 (14–4 Big East)Head coachMike BreyAssistant coaches Anthony Solomon Rod Balanis Martin Ingelsby Home arenaPurcell Pavilion at the Joyce CenterSeasons← 2009–102011–12 → 2010–11 Big East men's basketball standings vte Conf Overall Team W ...

British R&B and soul music singer (born 1952) This biography of a living person needs additional citations for verification. Please help by adding reliable sources. Contentious material about living persons that is unsourced or poorly sourced must be removed immediately from the article and its talk page, especially if potentially libelous.Find sources: Maxine Nightingale – news · newspapers · books · scholar · JSTOR (March 2018) (Learn how and whe...

University in Kobe, Japan Not to be confused with Kobe College. Kobe University神戸大学Latin: Kobienasis UniversitasOther nameShindai (神大)Motto真摯・自由・協同Motto in EnglishIntegrity – Freedom – CooperationTypePublic (National)EstablishedMarch 1902 (as the Kobe Higher Commercial School)(31 May 1949 at reformation of educational system) PresidentMasato FujisawaAcademic staff1,288 full-time (May 1, 2022)[1]Students15,870 (May 1, 2022)[1]Undergraduates1...

Caltanissettacomune Caltanissetta – VedutaPanorama della città dalla cima del monte San Giuliano LocalizzazioneStato Italia Regione Sicilia Libero consorzio comunale Caltanissetta AmministrazioneSindacoRoberto Gambino (M5S) dal 15-5-2019 TerritorioCoordinate37°29′29.3″N 14°03′44.8″E37°29′29.3″N, 14°03′44.8″E (Caltanissetta) Altitudine568 m s.l.m. Superficie421,25 km² Abitanti58 342[1] (31-12-2023) Densità138,5 ab....
علم جمهورية الكونغو الديمقراطية ألوان أزرق سماوي أصفر أحمر الاعتماد 20 فبراير 2006 الاختصاص جمهورية الكونغو الديمقراطية تعديل مصدري - تعديل علم جمهورية الكونغو الديمقراطية (بالفرنسية: drapeau de la république démocratique du Congo) أعتمد علم جمهورية الكونغو الديمقراطية في 18...

Dalam nama Tionghoa ini, nama keluarganya adalah Wu. Wu Faxian吴法宪Wu Faxian pada 1955 Panglima Angkatan Udara TPR ke-2Masa jabatanMei 1965 – September 1971PendahuluLiu YalouPenggantiMa Ning (lowong sampai 1973)Komisar Politik Angkatan Udara TPR ke-2Masa jabatan1957–1965PendahuluXiao HuaPenggantiYu Lijin Informasi pribadiLahir(1915-08-25)25 Agustus 1915Kabupaten Yongfeng, Jiangxi, TiongkokMeninggal17 Oktober 2004(2004-10-17) (umur 89)Jinan, Shandong, TiongkokPartai polit...

Untuk the 1935 film, lihat The Affair of Susan. The Affairs of SusanJoan Fontaine dalam The Affairs of SusanSutradaraWilliam A. SeiterProduserHal WallisSkenarioThomas MonroeLászló GörögRichard FlournoyCeritaThomas MonroeLászló GörögPemeranJoan FontaineGeorge BrentPenata musikFriedrich HollaenderSinematograferDavid AbelPenyuntingEda WarrenPerusahaanproduksiHal Wallis ProductionsDistributorParamount PicturesTanggal rilis 28 Maret 1945 (1945-03-28) (New York City) 08 Juli ...

Historic house in South Carolina, United States United States historic placeSouth Carolina Governor's MansionU.S. National Register of Historic PlacesU.S. Historic districtContributing property Show map of South CarolinaShow map of the United StatesLocation800 Richland St., Columbia, Richland County, South CarolinaCoordinates34°0′28″N 81°2′37″W / 34.00778°N 81.04361°W / 34.00778; -81.04361Area9 acres (3.6 ha)Built1855Architectattributed to George Edwar...

Senior rank in the armed forces This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Brigadier general – news · newspapers · books · scholar · JSTOR (January 2022) (Learn how and when to remove this message) Comparative military ranks Armies,air forces (non-Commonwealth) Navies, coast guards Air forces(Commonweal...
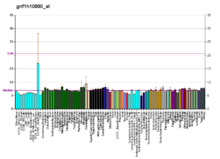
OR10K1 معرفات أسماء بديلة OR10K1, OR1-6, olfactory receptor family 10 subfamily K member 1 معرفات خارجية HomoloGene: 84605 GeneCards: 391109 علم الوجود الجيني وظائف جزيئية • G protein-coupled receptor activity• olfactory receptor activity• transmembrane signaling receptor activity• signal transducer activity مكونات خلوية • مكون تكاملي للغشاء• غشاء خلوي• غشاء عمليات حيوية • ...
この記事は検証可能な参考文献や出典が全く示されていないか、不十分です。 出典を追加して記事の信頼性向上にご協力ください。(このテンプレートの使い方)出典検索?: 長崎県立大学 – ニュース · 書籍 · スカラー · CiNii · J-STAGE · NDL · dlib.jp · ジャパンサーチ · TWL (2020年8月) この記事に雑多な内容を羅列した節がありま�...
