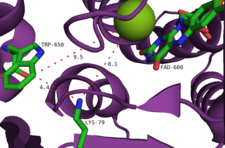
Tryptophan 7-halogenase
| |||||||||||||||||||||||||||||||||||||
Read other articles:

Andronikos II PalaiologosKaisar Andronikos II, lukisan fresko pada dinding biara di SerresKaisar dari Kekaisaran BizantiumBerkuasa1261 – 11 Desember 1282Penobatan1272PendahuluMikhael VIII Palaiologos (memerintah sendirian)PenerusDirinya sendiriBertahta bersamaMikhael VIII PalaiologosKaisar dari Kekaisaran BizantiumBerkuasa11 Desember 1282 – 1294PendahuluDirinya sendiri(bersama Mikhael VIII Palaiologos)PenerusDirinya sendiri(bersama Mikhael IX Palaiologos)Kaisar dari Kekaisaran BizantiumBe...

Biografi ini tidak memiliki sumber tepercaya sehingga isinya tidak dapat dipastikan. Bantu memperbaiki artikel ini dengan menambahkan sumber tepercaya. Materi kontroversial atau trivial yang sumbernya tidak memadai atau tidak bisa dipercaya harus segera dihapus.Cari sumber: Al-Mu'tashim Billah – berita · surat kabar · buku · cendekiawan · JSTOR (Pelajari cara dan kapan saatnya untuk menghapus pesan templat ini) Biografi ini memerlukan lebih banyak cata...

Village in SerbiaCaparić Цапарић (Serbian)Village (Selo)CaparićCoordinates: 44°10′N 19°28′E / 44.167°N 19.467°E / 44.167; 19.467Country SerbiaMunicipalityLjubovijaTime zoneUTC+1 (CET) • Summer (DST)UTC+2 (CEST) Caparić (Serbian Cyrillic: Цапарић) is a village in Serbia. It is situated in the Ljubovija municipality, in the Mačva District of Central Serbia. The village has a Serb ethnic majority. It had a population of 448 ...

العلاقات البحرينية الفلبينية البحرين الفلبين السفارات سفارة الفلبين في البحرين السفير : ساهيد قلانق العنوان : فيلا: 939 طريق: 3220 مجمع: 332 الماحوز قنصلية البحرين الفخرية في الفلبين السفير : أمابل س. أغويلوز العنوان : الدور �...

Unincorporated community in Virginia, United States Post office Randolph, formerly called Roanoke and Talcott, is a small unincorporated community in Charlotte County, Virginia, United States, near the Staunton River. Its elevation is 354 feet (108 m). The community is the home of Staunton River Battlefield State Park. Mulberry Hill and the Wade Archeological Site are listed on the National Register of Historic Places.[1] Climate The climate in this area is characterized by hot, humid...

For other uses, see Barrie (disambiguation). City in Ontario, CanadaBarrieCity (single-tier)City of BarrieFrom top, left to right: Downtown Barrie, MacLaren Art Centre, the CKVR-TV Tower, the Spirit Catcher, Sadlon Arena FlagCoat of armsLogoMotto: The People are the CityBarrieShow map of Southern OntarioBarrieShow map of Simcoe CountyCoordinates: 44°22′16″N 79°40′37″W / 44.37111°N 79.67694°W / 44.37111; -79.67694[1]CountryCanadaProvinceOntarioC...

Pour les articles homonymes, voir Joe Dassin (album). Joe DassinJoe Dassin vers 1970.BiographieNaissance 5 novembre 1938Brooklyn (New York, États-Unis)Décès 20 août 1980 (à 41 ans)Papeete (Polynésie française, France)Sépulture Hollywood Forever CemeteryNom dans la langue maternelle Joseph DassinNom de naissance Joseph Ira DassinSurnom Joe DassinNationalités américainefrançaiseFormation Collège de la littérature, des sciences et des arts de l'université du Michigan (en)Acti...

Choice of law in contract disputes Rome conventionConvention on the Law Applicable to Contractual ObligationsStates applying Rome instruments Rome I Regulation, Rome Convention Rome ConventionSigned19 June 1980LocationRomeEffective1 April 1991[1]Condition7 ratificationsPartiesall Member States of the European UnionDepositaryDirector-General of the Council of the European CommunitiesLanguagesDanish, Dutch, German, English, French, Irish and Italian (original) Th...

BazzarCompanyCirque du SoleilGenreContemporary circusShow typeTouring showDate of premiereNovember 14, 2018 (Mumbai, India)Other informationPreceded byCrystal (2017)Succeeded byAlegria: In a New Light (2019)Official website Bazzar is a touring show by Cirque du Soleil that premiered on 14 November 2018 in Mumbai, India. It is the company's 43rd production, and its first show to perform in India.[1][2][3] From India, it moved to Riyadh in Saudi Arabia.[4][5&...

سلمان واكسمان (بالإنجليزية: Selman Abraham Waksman) معلومات شخصية الميلاد 22 يوليو 1888 [1][2][3] الوفاة 16 أغسطس 1973 (85 سنة) [1][2][3] وودز هول الإقامة الولايات المتحدة مواطنة الولايات المتحدة (1916–) الإمبراطورية الروسية الاتحاد السوفيتي عضو في �...

Samson Siasia Nazionalità Nigeria Altezza 180 cm Peso 72 kg Calcio Ruolo Allenatore (ex attaccante) Termine carriera 2000 - giocatore CarrieraSquadre di club1 1982-1984 Julius Berger? (?)1985-1986 Flash Flamingoes? (?)1987 El-Kanemi Warriors? (?)1987-1993 Lokeren151 (31)1993-1995 Nantes-Atlantique40 (4)1995-1996 Tirsense15 (0)1996-1997 Al-Hilal? (?)1997-1998 Perth Glory22 (3)1998-2000 Hapoel Tzafririm Holon30 (12)Nazionale 1985 Nigeria U-20...

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: SMA Negeri 1 Saparua – berita · surat kabar · buku · cendekiawan · JSTOR Artikel ini perlu dikembangkan agar dapat memenuhi kriteria sebagai entri Wikipedia.Bantulah untuk mengembangkan artikel ini. Jika...

本表是動態列表,或許永遠不會完結。歡迎您參考可靠來源來查漏補缺。 潛伏於中華民國國軍中的中共間諜列表收錄根據公開資料來源,曾潛伏於中華民國國軍、被中國共產黨聲稱或承認,或者遭中華民國政府調查審判,為中華人民共和國和中國人民解放軍進行間諜行為的人物。以下列表以現今可查知時間為準,正確的間諜活動或洩漏機密時間可能早於或晚於以下所歸�...

Loggia dei Lanzi. Loggia dei Lanzi, juga disebut Loggia della Signoria, adalah sebuah bangunan yang terletak di salah satu sudut Piazza della Signoria di Firenze, Italia. Bangunan ini terletak bersebelahan dengan Galeri Uffizi. Loggia ini dibangun dari tahun 1376 hingga 1382 oleh Benci di Cione dan Simone di Francesco Talenti.[1] Perancangnya kemungkinan besar adalah Jacopo di Sione. Tujuan pendirian bangunan ini adalah untuk mengadakan pertemuan rakyat dan upacara-upacara resmi,[...

1939–1940 sci-fi fanzine by Ray Bradbury Futuria FantasiaCover of the first issueEditorRay BradburyCategoriesScience fictionFirst issue1939Final issue1940LanguageEnglish Futuria Fantasia was an American science fiction fanzine created by Ray Bradbury in 1938, when he was 18 years old. Though only four issues of the fanzine were published, its list of contributors included Hannes Bok, Forrest J. Ackerman, Henry Kuttner, Damon Knight, and Robert A. Heinlein. Background Since the 1930s, fanzin...

American army soldier (1896–1988) This article possibly contains original research. Please improve it by verifying the claims made and adding inline citations. Statements consisting only of original research should be removed. (November 2020) (Learn how and when to remove this message) Joseph F. AmbroseAmbrose in uniform during a homage to Vietnam War veterans, 1982BornJoseph Francis Ambrose(1896-05-24)May 24, 1896Joliet, Illinois, U.S.DiedMay 1, 1988 (aged 91)Joliet, Illinois, U.S.Resting ...

2014 UK local government election The Metropolitan Borough of Bolton shown within England. Elections to Bolton Metropolitan Borough Council were held on 22 May 2014, along with the European Parliament elections, 2014. One third of the council was up for election, with each successful candidate to serve a four-year term of office, expiring in 2018. 21 seats were contested, including 2 seats in the Horwich and Blackrod ward following the resignation of Labour councillor Lindsey Kell. The Labour...

Not to be confused with cerium. This article is about the chemical element. For the ancient city located in Cyprus, see Kourion. Chemical element, symbol Cm and atomic number 96Curium, 96CmCuriumPronunciation/ˈkjʊəriəm/ (KURE-ee-əm)Appearancesilvery metallic, glows purple in the darkMass number[247]Curium in the periodic table Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulfur Chlorine Argon P...

Scottish peerage Arms of the Earls of Glencairn as recorded in Brown's Peerage, 1834 Earl of Glencairn was a title in the Peerage of Scotland. It was created in 1488 for Alexander Cunningham, 1st Lord Kilmaurs (created 1450). The name was taken from the parish of Glencairn in Dumfriesshire so named for the Cairn Waters which run through it.[1] On the death of the fifteenth earl in 1796, there existing no original Letters Patent of the creation nor a given remainder in the various conf...

Bad GuyPoster promosi untuk Bad GuyGenreMelodramaSutradaraLee Hyung-minPemeranKim Nam-gilHan Ga-inKim Jae-wookOh Yeon-sooJung So-minPenata musikErica YK Jung (Chung Yea-kyung)Negara asalKorea SelatanBahasa asliKoreaJmlh. episode17ProduksiLokasi produksiKorea Nagoya, JepangDurasiRabu dan Kamis pukul 21:55 (WSK)Rumah produksiGood Story NHK[1]Rilis asliRilis26 Mei (2010-05-26) –05 Agustus 2010 (2010-08-05) Korean nameHangul나쁜 남자 Alih AksaraNappeun NamjaMcC...